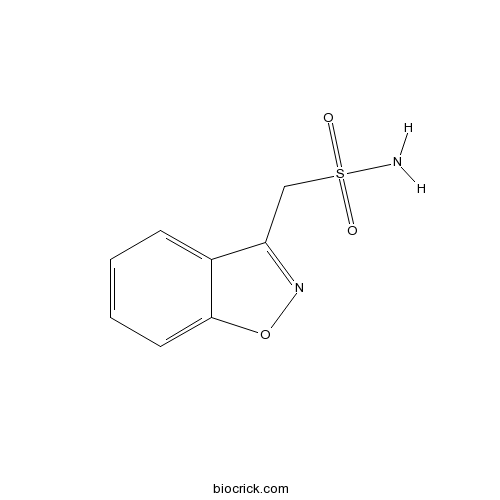
ZonisamideAntiepileptic with anticonvulsant and mechanistic effect CAS# 68291-97-4 |
- Mibefradil
Catalog No.:BCC1748
CAS No.:116644-53-2
- Mibefradil dihydrochloride
Catalog No.:BCC1749
CAS No.:116666-63-8
- Cilnidipine
Catalog No.:BCC1083
CAS No.:132203-70-4
- NNC 55-0396
Catalog No.:BCC1803
CAS No.:357400-13-6
- NP118809
Catalog No.:BCC1807
CAS No.:41332-24-5
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 68291-97-4 | SDF | Download SDF |
| PubChem ID | 5734 | Appearance | Powder |
| Formula | C8H8N2O3S | M.Wt | 212.23 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | AD 810, CI 912 | ||
| Solubility | DMSO : ≥ 100 mg/mL (471.19 mM) H2O : 0.67 mg/mL (3.16 mM; Need ultrasonic) *"≥" means soluble, but saturation unknown. | ||
| Chemical Name | 1,2-benzoxazol-3-ylmethanesulfonamide | ||
| SMILES | C1=CC=C2C(=C1)C(=NO2)CS(=O)(=O)N | ||
| Standard InChIKey | UBQNRHZMVUUOMG-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C8H8N2O3S/c9-14(11,12)5-7-6-3-1-2-4-8(6)13-10-7/h1-4H,5H2,(H2,9,11,12) | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Antiepileptic that possesses a broad spectrum anticonvulsant and mechanistic profile. Blocks voltage-sensitive Na+ and T-type Ca2+ channels, stimulates BKCa channels, modulates GABA, glutamate and monoamine neurotransmission, inhibits lipid peroxidation and scavenges hydroxyl and nitric oxide free radicals. Displays neuroprotective and antiParkinsonian activity. |

Zonisamide Dilution Calculator

Zonisamide Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 4.7119 mL | 23.5593 mL | 47.1187 mL | 94.2374 mL | 117.7967 mL |
| 5 mM | 0.9424 mL | 4.7119 mL | 9.4237 mL | 18.8475 mL | 23.5593 mL |
| 10 mM | 0.4712 mL | 2.3559 mL | 4.7119 mL | 9.4237 mL | 11.7797 mL |
| 50 mM | 0.0942 mL | 0.4712 mL | 0.9424 mL | 1.8847 mL | 2.3559 mL |
| 100 mM | 0.0471 mL | 0.2356 mL | 0.4712 mL | 0.9424 mL | 1.178 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Antiepileptic that possesses a broad spectrum anticonvulsant and mechanistic profile. Blocks voltage-sensitive Na+ and T-type Ca2+ channels, stimulates BKCa channels, modulates GABA, glutamate and monoamine neurotransmissi
- Boc-Arg(Mts)-OH.CHA
Catalog No.:BCC3045
CAS No.:68262-71-5
- (R)-Oxiracetam
Catalog No.:BCC4169
CAS No.:68252-28-8
- 2-Hydroxy-3-(hydroxymethyl)anthraquinone
Catalog No.:BCN1380
CAS No.:68243-30-1
- 6-Prenylnaringenin
Catalog No.:BCN2999
CAS No.:68236-13-5
- 6,8-Diprenylnaringenin
Catalog No.:BCN3000
CAS No.:68236-11-3
- BRL 44408 maleate
Catalog No.:BCC6948
CAS No.:681806-46-2
- Isoliensinine
Catalog No.:BCN6331
CAS No.:6817-41-0
- Nortetraphyllicine
Catalog No.:BCN4240
CAS No.:68160-76-9
- 1-O-Acetyl britannilactone
Catalog No.:BCN2365
CAS No.:681457-46-5
- Jujuboside B1
Catalog No.:BCN3881
CAS No.:68144-21-8
- Humic acid sodium salt
Catalog No.:BCN1284
CAS No.:68131-04-4
- Diosgenyl-3-di-β-O-glucopyranoside
Catalog No.:BCC8168
CAS No.:68127-19-5
- Zonisamide sodium
Catalog No.:BCC4240
CAS No.:68291-98-5
- Planinin
Catalog No.:BCC8981
CAS No.:68296-27-5
- Amlexanox ethyl ester
Catalog No.:BCC8818
CAS No.:68301-99-5
- Amlexanox
Catalog No.:BCC8817
CAS No.:68302-57-8
- Aristolone
Catalog No.:BCN4039
CAS No.:6831-17-0
- Imbricatolic acid
Catalog No.:BCN4241
CAS No.:6832-60-6
- Nonactin
Catalog No.:BCC7388
CAS No.:6833-84-7
- 1-Methyl-2-nonylquinolin-4(1H)-one
Catalog No.:BCN6585
CAS No.:68353-24-2
- 23,24-dihydroisocucurbitacin B
Catalog No.:BCN7876
CAS No.:68354-21-2
- Deltaline
Catalog No.:BCN5404
CAS No.:6836-11-9
- 7-Methoxy-1-tetralone
Catalog No.:BCN2241
CAS No.:6836-19-7
- 7-Methoxy-1-naphthaleneacetic acid ethyl ester
Catalog No.:BCN1379
CAS No.:6836-21-1
Effect of topiramate and zonisamide on fMRI cognitive networks.[Pubmed:28213372]
Neurology. 2017 Mar 21;88(12):1165-1171.
OBJECTIVE: To investigate the effects of topiramate (TPM), Zonisamide (ZNS), and levetiracetam (LEV) on cognitive network activations in patients with focal epilepsy using an fMRI language task. METHODS: In a retrospective, cross-sectional study, we identified patients from our clinical database of verbal fluency fMRI studies who were treated with either TPM (n = 32) or ZNS (n = 51). We matched 62 patients for clinical measures who took LEV but not TPM or ZNS. We entered antiepileptic comedications as nuisance variables and compared out-of-scanner psychometric measures for verbal fluency and working memory between groups. RESULTS: Out-of-scanner psychometric data showed overall poorer performance for TPM compared to ZNS and LEV and poorer working memory performance in ZNS-treated patients compared to LEV-treated patients. We found common fMRI effects in patients taking ZNS and TPM, with decreased activations in cognitive frontal and parietal lobe networks compared to those taking LEV. Impaired deactivation was seen only with TPM. CONCLUSIONS: Our findings suggest that TPM and ZNS are associated with similar dysfunctions of frontal and parietal cognitive networks, which are associated with impaired performance. TPM is also associated with impaired attenuation of language-associated deactivation. These studies imply medication-specific effects on the functional neuroanatomy of language and working memory networks. CLASSIFICATION OF EVIDENCE: This study provides Class III evidence that in patients with focal epilepsy, TPM and ZNS compared to LEV lead to disruption of language and working memory networks.
The Impact of Zonisamide on the Development and Course of Alcohol Dependence in Rabbits. A pharmaco-EEG study.[Pubmed:28339635]
Alcohol Alcohol. 2017 May 1;52(3):282-288.
Aims: Zonisamide is a new anti-epileptic drug whose mechanism of action is associated with neurotransmission systems also involved in the pathogenesis of addiction. Recently, the role of memory processes and the hippocampus (Hp) is underlined in dependence. In our previous study, we determined that Zonisamide decreases changes in hippocampal bioelectric activity induced by a single dose of ethanol. Methods: This study uses a pharmaco-EEG method to examine the impact of Zonisamide on the development and course of alcohol dependence in rabbits. Quantitative changes in EEG were observed in the midbrain reticular formation, Hp and frontal cortex. Zonisamide was administered p.o. once a day at dose of 30 mg/kg/day during the entire experiment. Solutions with increasing concentrations of ethanol were administered for 6 weeks, followed by a 2-week period of abstinence. Results: The long-term administration of ethanol caused characteristic changes in rabbit EEG recordings, which were associated with a shift toward lower frequencies resulting in a depressive effect on the bioelectric activity of selected brain structures. Co-administration of Zonisamide and ethanol caused a reduction of ethanol-induced alterations. Changes in EEG recordings were different during period of abstinence and were associated with potent shift toward the high frequencies. Zonisamide significantly decreased encephalographic features of neuronal hyperactivity when administered during the abstinence. Conclusion: Zonisamide decreases ethanol- and abstinence-induced changes in the EEG recordings. These effects may be a significant part of drug's mechanism of action in alcohol addiction therapy. Short Summary: A pharmaco-EEG method was used to determine the influence of a new anti-epileptic drug Zonisamide on the development and course of alcohol dependence in rabbits. The drug co-administered with ethanol decreased alcohol-induced changes in selected brain structures. Zonisamide also decreases abstinence-induced changes in the EEG recordings.
The Use of Zonisamide for the Treatment of Psychiatric Disorders: A Systematic Review.[Pubmed:28195838]
Clin Neuropharmacol. 2017 Mar/Apr;40(2):85-92.
OBJECTIVE: Traditional pharmacotherapy has undoubtedly improved the outcome of patients with psychiatric disorders, but partial efficacy or poor tolerability persists in a number of these subjects. Among different compounds, Zonisamide has been used to address unmet needs of standard pharmacotherapy. The purpose of the present article is to provide a review about the use of Zonisamide for the treatment of psychiatric conditions. METHODS: A research in the main database sources has been conducted to obtain an overview of the use of Zonisamide in psychiatric disorders or associated conditions (obesity and smoking cessation). RESULTS: Most available data indicate the possible effectiveness of Zonisamide for the treatment of acute phases of bipolar disorder, binge-eating disorder (BED), alcohol misuse, and obesity. A further assessment of the safety and tolerability of Zonisamide is made necessary by the fact that, with the exception of BED, for all other disorders at least some data come from studies with combined pharmacological therapies. CONCLUSIONS: Zonisamide may have some utility, especially as an adjunctive therapy, for the management of acute phases and weight gain in bipolar disorder and for prevention of alcohol misuse. Preliminary evidence indicates Zonisamide as a candidate compound for the treatment of BED and obesity. However, open-label design and small sample sizes of most available studies prevent from drawing sound conclusions about the utility of this compound in psychiatry.
Combination Therapy with Zonisamide and Antiparkinson Drugs for Parkinson's Disease: A Meta-Analysis.[Pubmed:28157097]
J Alzheimers Dis. 2017;56(4):1229-1239.
BACKGROUND: There is uncertainty about the efficacy and tolerability of Zonisamide for Parkinson's disease (PD). OBJECTIVE: We performed a meta-analysis of Zonisamide treatment in PD patients who received antiparkinson drugs such as levodopa. METHODS: The primary outcome measures were the Unified Parkinson's Disease Rating Scale (UPDRS) Part III scores, wearing-off time, and discontinuation rate due to all causes. Secondary outcome measures were UPDRS total and subscale scores; discontinuation rates due to adverse events, inefficacy, and death; and individual adverse events. RESULTS: Four randomized placebo-controlled trials including 1,068 PD patients were analyzed. All studies were conducted in Japan. UPDRS Part III scores were significantly lower with Zonisamide than with placebo (weighted mean difference [WMD], -2.56; 95% confidence interval [CI]; -4.20 to -0.92; p = 0.002). Further, Zonisamide significantly decreased the wearing-off time compared with placebo (standardized mean difference, -0.24; 95% CI, -0.39 to -0.09; p = 0.001). Discontinuation rates due to all causes were similar between the Zonisamide and placebo groups (risk ratio, 1.29; 95% CI, 0.90 to 1.84; p = 0.16). While Zonisamide also decreased both UPDRS Part II (off-time) and UPDRS total scores compared to placebo (UPDRS Part II [off-time] scores: WMD, -0.79; UPDRS total scores: WMD, -2.51), there were no significant differences in other secondary outcomes between the two groups. CONCLUSIONS: Our results suggested that Zonisamide combination therapy was beneficial in treating motor symptoms in PD patients receiving antiparkinson drugs and was well tolerated in Japanese patients. Future studies in populations other than the Japanese are needed.
Activation by zonisamide, a newer antiepileptic drug, of large-conductance calcium-activated potassium channel in differentiated hippocampal neuron-derived H19-7 cells.[Pubmed:17255467]
J Pharmacol Exp Ther. 2007 Apr;321(1):98-106.
Zonisamide (ZNS; 3-sulfamoylmethyl-1,2-benzisoxazole), as one of the newer antiepileptic drugs, has been demonstrated its broad-spectrum clinical efficacy on various neuropsychiatric disorders. However, little is known regarding the mechanism of ZNS actions on ion currents in neurons. We thus investigated its effect on ion currents in differentiated hippocampal 19-7 cells. In whole-cell configuration of patch-clamp technology, the ZNS (30 microM) reversibly increased the amplitude of K+ outward currents, and paxilline (1 microM) was effective in suppressing the ZNS-induced increase of K+ outward currents. In inside-out configuration, ZNS (30 microM) applied to the intracellular face of the membrane did not alter single-channel conductance; however, it did enhance the activity of large-conductance Ca2+-activated K+ (BK(Ca)) channels primarily by decreasing mean closed time. In addition, the EC50 value for ZNS-stimulated BK(Ca) channels was 34 microM. This drug caused a left shift in the activation curve of BK(Ca) channels, with no change in the gating charge of these channels. Moreover, ZNS at a concentration greater than 100 microM also reduced the amplitude of A-type K+ current in these cells. A simulation modeling based on hippocampal CA3 pyramidal neurons (Pinsky-Rinzel model) was also analyzed to investigate the inhibitory effect of ZNS on the firing of simulated action potentials. Taken together, this study suggests that, in hippocampal neurons during the exposure to ZNS, the ZNS-mediated effects on BK(Ca) channels and A-type K+ current could be potential mechanisms through which it affects neuronal excitability.
Novel dopamine releasing response of an anti-convulsant agent with possible anti-Parkinson's activity.[Pubmed:15168218]
J Neural Transm (Vienna). 2004 Jun;111(6):713-24.
We used cerebral microdialysis to assess the ability of the anticonvulsant drug Zonisamide (ZNS) to release striatal dopamine in 6-hydroxydopamine nigrotomized rats. Following exogeneously administered ZNS we measured dopamine (DA), 3,4-dihydroxyphenylacetic acid (DOPAC) and homovanillic acid (HVA) levels in striatal dialysates obtained from the ipsilateral side of the nigrotomy. ZNS administration alone had no effect on levels of DA and its metabolites or rotational behavior. Administration of carbidopa-levodopa alone led to small but insignificant increases in rotational behavior contralateral to the side of the nigrotomy but no corresponding increases in indices of striatal catecholamine release. In contrast, if animals were preloaded with carbidopa and ZNS was co-administered with levodopa 30 minutes later significant increases in contralateral rotational behavior occurred within 20 minutes of ZNS-levodopa injection that lasted for at least 90 minutes. In contrast to the uniform rotational behavioral responses observed in all our nigrotomized animals, less than half demonstrated neurochemical evidence of DA release. In these "responder" animals DOPAC levels increased 300% following carbidopa-levodopa-ZNS administration. We conclude that these results support previously reported findings and provide additional evidence that the anticonvulsant ZNS appears to possess anti-Parkinson's properties. ZNS could therefore be a novel agent for the treatment of PD that could delay the use of or reduce the amount of levodopa needed to treat patients with PD.
Zonisamide: a new antiepileptic drug.[Pubmed:14704463]
Pol J Pharmacol. 2003 Sep-Oct;55(5):683-9.
Although the significant progress in pharmacotherapy of epilepsy during last decade was achieved, about one third of patients are resistant to the current treatment. When the monotherapy is not efficient, the polytherapy should be applied. Zonisamide (ZNS) is a new antiepileptic drug (AED) efficient in treating refractory epilepsy. Its efficacy in different types of seizures was confirmed in various animal studies as well as in clinical conditions. ZNS inhibits voltage-dependent Na(+) channels and Ca(2+) channels of T-type. The drug influences also monoamine neurotransmission and exhibits free radical scavenging properties. ZNS has a linear and favorable pharmacokinetics with excellent oral bioavailability. Furthermore, ZNS treatment, compared to other anticonvulsants, is relatively safe and well tolerated. Since ZNS is often used in polytherapy, its interactions with other AEDs seem to be of particular importance. However, the experimental data are rather inconsistent and further studies are necessary to elucidate exact effects of coadministration of ZNS with other AEDs. Recently, the clinical and experimental studies have suggested some new indications for ZNS administration, as mania, neuropathic pain, Parkinson's disease or migraine prophylaxis. Nowadays, it is also well established that ZNS exerts neuroprotective properties.
In vivo evaluation of hippocampal anti-oxidant ability of zonisamide in rats.[Pubmed:11055748]
Neurochem Res. 2000 Aug;25(8):1107-11.
We evaluated the anti-oxidant property of Zonisamide (ZNS) in the rat brain under freely moving conditions by means of in vivo microdialysis of two exogenous nitroxide radicals, 3-carbamoyl-2,2,5,5-tetramethylpyrrolidine-1-oxyl (carbamoyl-PROXYL) and 3-methoxy carbonyl-2,2,5,5-tetramethylpyrrolidine-1-oxyl (PCAM). Time-dependent changes in the signal intensities of these exogenous nitroxide radicals obtained from the hippocampal perfusates were observed using an X-band ESR spectrometer at 20-min intervals. The ESR signal intensities of nitroxide radicals decreased exponentially in all animals, which indicates that their half-life could be used as a parameter to estimate the decay rate of nitroxide radicals. Nitroxide radicals lose their paramagnetism when exposed to reductants in a biological system. Thus, half-life reflects the in vivo reducing ability. Although the half-life of carbamoyl-PROXYL, which could not pass the blood-brain barrier (BBB), was not changed when compared with the controls, pre-treatment with ZNS significantly shortened the half-life of PCAM, which could pass through the BBB. These findings suggest that the ZNS-induced increase in reducing ability did not occur within the extracellular space, but rather mainly at the neural cell membrane. This study is the first in vivo evaluation of the reducing ability of ZNS in freely moving animals.


