(R)-OxiracetamCAS# 68252-28-8 |
- Anguizole
Catalog No.:BCC1365
CAS No.:442666-98-0
- Asunaprevir (BMS-650032)
Catalog No.:BCC1374
CAS No.:630420-16-5
- Balapiravir
Catalog No.:BCC1396
CAS No.:690270-29-2
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 68252-28-8 | SDF | Download SDF |
| PubChem ID | 3051965 | Appearance | Powder |
| Formula | C6H10N2O3 | M.Wt | 158.16 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | (R)-(+)-Oxiracetam | ||
| Solubility | H2O : ≥ 50 mg/mL (316.14 mM) *"≥" means soluble, but saturation unknown. | ||
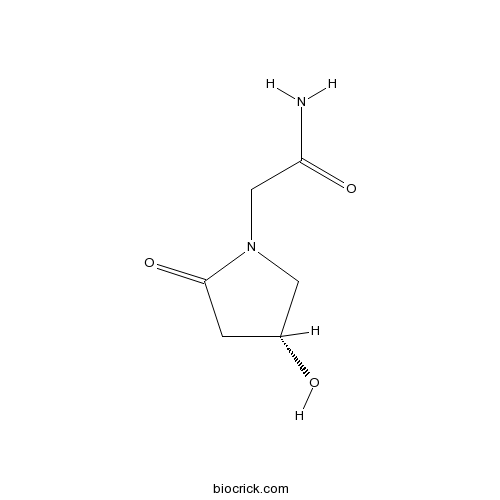
| Chemical Name | 2-[(4R)-4-hydroxy-2-oxopyrrolidin-1-yl]acetamide | ||
| SMILES | C1C(CN(C1=O)CC(=O)N)O | ||
| Standard InChIKey | IHLAQQPQKRMGSS-SCSAIBSYSA-N | ||
| Standard InChI | InChI=1S/C6H10N2O3/c7-5(10)3-8-2-4(9)1-6(8)11/h4,9H,1-3H2,(H2,7,10)/t4-/m1/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | (R)-Oxiracetam is the (R)-enantiomer of the nootropic drug oxiracetam. Oxiracetam (ISF 2522) is a nootropic drug of the racetam family and stimulant.
IC50 value:
Target: nootropic drug
Oxiracetam (ISF 2522) is a nootropic drug of the racetam family and stimulant.[1][2] Several animal studies suggest that the substance is safe even when high doses are consumed for a long period of time.[medical citation needed] However, the mechanism of action of the racetam drug family is still a matter of research. Oxiracetam is not approved by Food and Drug Administration for any medical use in the United States. References: | |||||

(R)-Oxiracetam Dilution Calculator

(R)-Oxiracetam Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 6.3227 mL | 31.6136 mL | 63.2271 mL | 126.4542 mL | 158.0678 mL |
| 5 mM | 1.2645 mL | 6.3227 mL | 12.6454 mL | 25.2908 mL | 31.6136 mL |
| 10 mM | 0.6323 mL | 3.1614 mL | 6.3227 mL | 12.6454 mL | 15.8068 mL |
| 50 mM | 0.1265 mL | 0.6323 mL | 1.2645 mL | 2.5291 mL | 3.1614 mL |
| 100 mM | 0.0632 mL | 0.3161 mL | 0.6323 mL | 1.2645 mL | 1.5807 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
(R)-Oxiracetam is the (R)-enantiomer of the nootropic drug oxiracetam. Oxiracetam (ISF 2522) is a nootropic drug of the racetam family and stimulant.
- 2-Hydroxy-3-(hydroxymethyl)anthraquinone
Catalog No.:BCN1380
CAS No.:68243-30-1
- 6-Prenylnaringenin
Catalog No.:BCN2999
CAS No.:68236-13-5
- 6,8-Diprenylnaringenin
Catalog No.:BCN3000
CAS No.:68236-11-3
- BRL 44408 maleate
Catalog No.:BCC6948
CAS No.:681806-46-2
- Isoliensinine
Catalog No.:BCN6331
CAS No.:6817-41-0
- Nortetraphyllicine
Catalog No.:BCN4240
CAS No.:68160-76-9
- 1-O-Acetyl britannilactone
Catalog No.:BCN2365
CAS No.:681457-46-5
- Jujuboside B1
Catalog No.:BCN3881
CAS No.:68144-21-8
- Humic acid sodium salt
Catalog No.:BCN1284
CAS No.:68131-04-4
- Diosgenyl-3-di-β-O-glucopyranoside
Catalog No.:BCC8168
CAS No.:68127-19-5
- Astressin 2B
Catalog No.:BCC5906
CAS No.:681260-70-8
- Chonglou Saponin VII
Catalog No.:BCN4239
CAS No.:68124-04-9
- Boc-Arg(Mts)-OH.CHA
Catalog No.:BCC3045
CAS No.:68262-71-5
- Zonisamide
Catalog No.:BCC2512
CAS No.:68291-97-4
- Zonisamide sodium
Catalog No.:BCC4240
CAS No.:68291-98-5
- Planinin
Catalog No.:BCC8981
CAS No.:68296-27-5
- Amlexanox ethyl ester
Catalog No.:BCC8818
CAS No.:68301-99-5
- Amlexanox
Catalog No.:BCC8817
CAS No.:68302-57-8
- Aristolone
Catalog No.:BCN4039
CAS No.:6831-17-0
- Imbricatolic acid
Catalog No.:BCN4241
CAS No.:6832-60-6
- Nonactin
Catalog No.:BCC7388
CAS No.:6833-84-7
- 1-Methyl-2-nonylquinolin-4(1H)-one
Catalog No.:BCN6585
CAS No.:68353-24-2
- 23,24-dihydroisocucurbitacin B
Catalog No.:BCN7876
CAS No.:68354-21-2
- Deltaline
Catalog No.:BCN5404
CAS No.:6836-11-9
Pharmacokinetic comparisons of S-oxiracetam and R-oxiracetam in beagle dogs.[Pubmed:27279070]
Acta Pharm. 2016 Jun 1;66(2):279-87.
A pharmacokinetic comparison and conformational stability study of S-oxiracetam (S-ORT) and R-oxiracetam (R-ORT) in beagle dogs was used to investigate the possible mechanism of different effects of two oxiracetam enantiomers through a random crossover design. After drug administration to beagle dogs, blood samples were collected at different time points for pharmacokinetic analysis using the UPLC-ESI-MS/MS method. Parts of plasma samples were used for conformation transformation studies using a normal phase high performance liquid chromatographic (NP HPLC) method. The study showed that oxiracetam enantiomers maintained their original conformation when administered orally to beagle dogs. Concentrations of S-ORT were significantly higher than R-ORT 1.5 and 2 h after administration; the AUC0-infinity of S-ORT after oral administration tended to be higher than that of R-ORT, which showed that the different effects between S-ORT and R-ORT may be partly associated with their distinctive absorption at least.


