Astressin 2BSelective CRF2 antagonist CAS# 681260-70-8 |
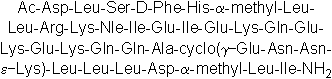
- G-749
Catalog No.:BCC4009
CAS No.:1457983-28-6
- Cabozantinib (XL184, BMS-907351)
Catalog No.:BCC1264
CAS No.:849217-68-1
- Amuvatinib (MP-470, HPK 56)
Catalog No.:BCC2258
CAS No.:850879-09-3
- TG101209
Catalog No.:BCC2198
CAS No.:936091-14-4
- Quizartinib (AC220)
Catalog No.:BCC2548
CAS No.:950769-58-1
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 681260-70-8 | SDF | Download SDF |
| PubChem ID | 56972208 | Appearance | Powder |
| Formula | C183H307N49O53 | M.Wt | 4041.69 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble to 1 mg/ml in water | ||
| Sequence | DLSFHLLRKXIEIEKQEKEKQQAENNKLLL (Modifications: Asp-1 = N-terminal Ac, Phe-4 = D-Phe, Leu-6 = α-methyl-Leu, X = Nle, Glu-24 = γ-Glu, Lys-27 = ε-Lys, Leu-32 = α-methyl-Leu, Lactam Bridge = Glu-24 - Lys-27) | ||
| SMILES | CCCCC(C(=O)NC(C(C)CC)C(=O)NC(CCC(=O)O)C(=O)NC(C(C)CC)C(=O)NC(CCC(=O)O)C(=O)NC(CCCCN)C(=O)NC(CCC(=O)N)C(=O)NC(CCC(=O)O)C(=O)NC(CCCCN)C(=O)NC(CCC(=O)O)C(=O)NC(CCCCN)C(=O)NC(CCC(=O)N)C(=O)NC(CCC(=O)N)C(=O)NC(C)C(=O)NC1CCC(=O)NCCCCC(NC(=O)C(NC(=O)C(NC1=O)CC(=O)N)CC(=O)N)C(=O)NC(CC(C)C)C(=O)NC(CC(C)C)C(=O)NC(CC(C)C)C(=O)NC(CC(=O)O)C(=O)NC(C)(CC(C)C)C(=O)NC(C(C)CC)C(=O)N)NC(=O)C(CCCCN)NC(=O)C(CCCNC(=N)N)NC(=O)C(CC(C)C)NC(=O)C(C)(CC(C)C)NC(=O)C(CC2=CNC=N2)NC(=O)C(CC3=CC=CC=C3)NC(=O)C(CO)NC(=O)C(CC(C)C)NC(=O)C(CC(=O)O)NC(=O)C | ||
| Standard InChIKey | IVIBPRHVUKMKSX-XJYAKNQHSA-N | ||
| Standard InChI | InChI=1S/C183H307N49O53/c1-26-30-47-106(203-151(256)107(48-34-39-70-184)204-155(260)112(53-44-75-198-181(194)195)209-169(274)125(80-96(13)14)227-179(284)182(24,87-97(15)16)231-175(280)127(82-105-89-196-91-199-105)222-170(275)126(81-104-45-32-31-33-46-104)221-174(279)132(90-233)226-168(273)124(79-95(11)12)220-173(278)130(85-143(249)250)201-103(23)234)163(268)228-146(100(20)28-3)178(283)216-120(61-69-142(247)248)164(269)229-147(101(21)29-4)177(282)215-119(60-68-141(245)246)161(266)207-109(50-36-41-72-186)153(258)212-115(56-64-135(190)237)158(263)214-118(59-67-140(243)244)160(265)206-110(51-37-42-73-187)154(259)213-117(58-66-139(241)242)159(264)205-108(49-35-40-71-185)152(257)211-114(55-63-134(189)236)157(262)210-113(54-62-133(188)235)150(255)200-102(22)149(254)202-116-57-65-138(240)197-74-43-38-52-111(208-171(276)128(83-136(191)238)224-172(277)129(84-137(192)239)223-162(116)267)156(261)217-121(76-92(5)6)165(270)218-122(77-93(7)8)166(271)219-123(78-94(9)10)167(272)225-131(86-144(251)252)176(281)232-183(25,88-98(17)18)180(285)230-145(148(193)253)99(19)27-2/h31-33,45-46,89,91-102,106-132,145-147,233H,26-30,34-44,47-88,90,184-187H2,1-25H3,(H2,188,235)(H2,189,236)(H2,190,237)(H2,191,238)(H2,192,239)(H2,193,253)(H,196,199)(H,197,240)(H,200,255)(H,201,234)(H,202,254)(H,203,256)(H,204,260)(H,205,264)(H,206,265)(H,207,266)(H,208,276)(H,209,274)(H,210,262)(H,211,257)(H,212,258)(H,213,259)(H,214,263)(H,215,282)(H,216,283)(H,217,261)(H,218,270)(H,219,271)(H,220,278)(H,221,279)(H,222,275)(H,223,267)(H,224,277)(H,225,272)(H,226,273)(H,227,284)(H,228,268)(H,229,269)(H,230,285)(H,231,280)(H,232,281)(H,241,242)(H,243,244)(H,245,246)(H,247,248)(H,249,250)(H,251,252)(H4,194,195,198)/t99-,100-,101-,102-,106-,107-,108-,109-,110-,111-,112-,113-,114-,115-,116-,117-,118-,119-,120-,121-,122-,123-,124-,125-,126+,127-,128-,129-,130-,131-,132-,145-,146-,147-,182-,183-/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Potent and selective corticotropin-releasing factor receptor 2 (CRF2) antagonist (IC50 values are 1.3 and > 500 nM for CRF2 and CRF1 respectively). Antagonizes CRF2-mediated inhibition of gastric emptying. |

Astressin 2B Dilution Calculator

Astressin 2B Molarity Calculator

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Chonglou Saponin VII
Catalog No.:BCN4239
CAS No.:68124-04-9
- Royleanone
Catalog No.:BCN3917
CAS No.:6812-87-9
- Roburic acid
Catalog No.:BCN5385
CAS No.:6812-81-3
- JNJ-1661010
Catalog No.:BCC2315
CAS No.:681136-29-8
- AICAR phosphate
Catalog No.:BCC4220
CAS No.:681006-28-0
- 13-Dehydroxyindaconintine
Catalog No.:BCN8403
CAS No.:681-18-9
- Bepridil hydrochloride
Catalog No.:BCC7864
CAS No.:68099-86-5
- 4',5,7-Trihydroxy-6-prenylflavone
Catalog No.:BCN4238
CAS No.:68097-13-2
- Boc-Phe(4-Cl)-OH
Catalog No.:BCC3172
CAS No.:68090-88-0
- Norfloxacin hydrochloride
Catalog No.:BCC4230
CAS No.:68077-27-0
- Trifolirhizin
Catalog No.:BCN4237
CAS No.:6807-83-6
- Megastigm-7-ene-3,5,6,9-tetraol
Catalog No.:BCN5169
CAS No.:680617-50-9
- Diosgenyl-3-di-β-O-glucopyranoside
Catalog No.:BCC8168
CAS No.:68127-19-5
- Humic acid sodium salt
Catalog No.:BCN1284
CAS No.:68131-04-4
- Jujuboside B1
Catalog No.:BCN3881
CAS No.:68144-21-8
- 1-O-Acetyl britannilactone
Catalog No.:BCN2365
CAS No.:681457-46-5
- Nortetraphyllicine
Catalog No.:BCN4240
CAS No.:68160-76-9
- Isoliensinine
Catalog No.:BCN6331
CAS No.:6817-41-0
- BRL 44408 maleate
Catalog No.:BCC6948
CAS No.:681806-46-2
- 6,8-Diprenylnaringenin
Catalog No.:BCN3000
CAS No.:68236-11-3
- 6-Prenylnaringenin
Catalog No.:BCN2999
CAS No.:68236-13-5
- 2-Hydroxy-3-(hydroxymethyl)anthraquinone
Catalog No.:BCN1380
CAS No.:68243-30-1
- (R)-Oxiracetam
Catalog No.:BCC4169
CAS No.:68252-28-8
- Boc-Arg(Mts)-OH.CHA
Catalog No.:BCC3045
CAS No.:68262-71-5
Corticotropin-Releasing Factor Receptors Modulate Oxytocin Release in the Dorsolateral Bed Nucleus of the Stria Terminalis (BNST) in Male Rats.[Pubmed:29618970]
Front Neurosci. 2018 Mar 21;12:183.
The neuropeptide oxytocin (OT) plays an important role in the regulation of social and anxiety-like behavior. Our previous studies have shown that OT neurons send projections from the hypothalamus to the dorsolateral bed nucleus of the stria terminalis (BNSTdl), a forebrain region critically involved in the modulation of anxiety-like behavior. Importantly, these OT terminals in the BNSTdl express presynaptic corticotropin releasing factor (CRF) receptor type 2 (CRFR2). This suggests that CRFR2 might be involved in the modulation of OT release. To test this hypothesis, we measured OT content in microdialysates collected from the BNSTdl of freely-moving male Sprague-Dawley rats following the administration of a selective CRFR2 agonist (Urocortin 3) or antagonist (Astressin 2B, As2B). To determine if type 1 CRF receptors (CRFR1) are also involved, we used selective CRFR1 antagonist (NBI35965) as well as CRF, a putative ligand of both CRFR1 and CRFR2. All compounds were delivered directly into the BNSTdl via reverse dialysis. OT content in the microdialysates was measured with highly sensitive and selective radioimmunoassay. Blocking CRFR2 with As2B caused an increase in OT content in BNSTdl microdialysates, whereas CRFR2 activation by Urocortin 3 did not have an effect. The As2B-induced increase in OT release was blocked by application of the CRFR1 antagonist demonstrating that the effect was dependent on CRFR1 transmission. Interestingly, CRF alone caused a delayed increase in OT content in BNSTdl microdialysates, which was dependent on CRF2 but not CRF1 receptors. Our results suggest that members of the CRF peptide family modulate OT release in the BNSTdl via a fine-tuned mechanism that involves both CRFR1 and CRFR2. Further exploration of mechanisms by which endogenous OT system is modulated by CRF peptide family is needed to better understand the role of these neuropeptides in the regulation of anxiety and the stress response.
CRF modulates glutamate transmission in the central amygdala of naive and ethanol-dependent rats.[Pubmed:28807676]
Neuropharmacology. 2017 Oct;125:418-428.
Corticotropin-releasing factor (CRF) signaling in the central nucleus of the amygdala (CeA) is hypothesized to drive the development of alcohol dependence, as it regulates ethanol intake and several anxiogenic behaviors linked to withdrawal. Excitatory glutamatergic neurotransmission contributes to alcohol reinforcement, tolerance and dependence. Therefore, in this study we used in vitro slice electrophysiology to investigate the effects of CRF and its receptor subtype (CRF1 and CRF2) antagonists on both evoked and spontaneous action potential-independent glutamatergic transmission in the CeA of naive and ethanol-dependent Sprague-Dawley rats. We found that CRF (25-200 nM) concentration-dependently diminished evoked compound excitatory postsynaptic potentials (EPSPs), but increased miniature excitatory postsynaptic current (mEPSC) frequencies similarly in CeA neurons of both naive and ethanol-dependent rats, indicating reduced evoked glutamatergic responses and enhanced vesicular glutamate release, respectively. This CRF-induced vesicular glutamate release was prevented by the CRF1/2 antagonist (Astressin B) and the CRF1 antagonist (R121919), but not by the CRF2 antagonist (Astressin 2B). Similarly, CRF's effects on evoked glutamatergic responses were completely blocked by CRF1 antagonism, but only slightly decreased in the presence of the CRF2 antagonist. Moreover, CRF1 antagonism reveals a tonic facilitation of vesicular glutamate, whereas the CRF2 antagonism revealed a tonic inhibition of vesicular glutamate release. Collectively our data show that CRF primarily acts at presynaptic CRF1 to produce opposite effects on CeA evoked and spontaneous glutamate release and that the CRF system modulates CeA glutamatergic synapses throughout the development of alcohol dependence.
Corticotropin-releasing hormone modulates airway vagal preganglionic neurons of Sprague-Dawley rats at multiple synaptic sites via activation of its type 1 receptors: Implications for stress-associated airway vagal excitation.[Pubmed:28499969]
Neuroscience. 2017 Jul 4;355:101-112.
Corticotropin-releasing hormone release is the final common pathway of stress-associated neuroendocrine responses. This study tested how corticotropin-releasing hormone modulates airway vagal preganglionic neurons. Airway vagal preganglionic neurons in neonatal rats were retrogradely labeled with fluorescent dye and identified in medullary slices, and their responses to corticotropin-releasing hormone (200nmolL(-1)) were examined using whole-cell patch clamp. The results show that under current clamp, corticotropin-releasing hormone (200nmolL(-1)) depolarized airway vagal preganglionic neurons and significantly increased the rate of their spontaneous firing. Under voltage clamp, corticotropin-releasing hormone caused a tonic inward current and significantly facilitated the spontaneous glutamatergic and GABAergic inputs of these neurons. Corticotropin-releasing hormone had no impact on the spontaneous glycinergic inputs of these neurons. In the preexistence of tetrodotoxin (1mumolL(-1)), corticotropin-releasing hormone had no impact on the miniature excitatory or inhibitory postsynaptic currents, but still induced a tonic inward current and significantly increased the input resistance. The responses induced by corticotropin-releasing hormone were prevented by Antalarmin hydrochloride (50mumolL(-1)), an antagonist of type 1 corticotropin-releasing hormone receptors, but insensitive to Astressin 2B (200nmolL(-1)), an antagonist of type 2 corticotropin-releasing hormone receptors. These results suggest that corticotropin-releasing hormone excites airway vagal preganglionic neurons via activation of its type 1 receptors at multiple sites, which includes a direct postsynaptic excitatory action and presynaptic facilitation of both glutamatergic and GABAergic inputs. In stress, corticotropin-releasing hormone might be able to activate the airway vagal nerves and, consequently, participate in induction or exacerbation of airway disorders.
The effect of lateral septum corticotropin-releasing factor receptor 2 activation on anxiety is modulated by stress.[Pubmed:16957071]
J Neurosci. 2006 Sep 6;26(36):9142-52.
Corticotropin-releasing factor (CRF), a 41 amino acid peptide, mediates endocrine, autonomic, and behavioral responses to stress. Whereas the CRF1 receptor appears to contribute to anxiety associated with stress, the role of the CRF2 receptor remains unclear and may depend on drug dose, brain location, or testing environment. Results involving treatments with selective CRF2 receptor agonists or antagonists and the behavior of CRF2 receptor knock-out mice suggest both anxiogenic and anxiolytic effects of CRF2 receptor activation. The present study tested the hypothesis that the effect of CRF2 receptor activation on anxiety depends on the stress level of the animal. The selective CRF2 receptor agonist urocortin 2 was infused into the lateral septum of mice under low- or high-stress (30 min of immobilization) testing conditions, and then behavior in the light-dark box, open-field, and novel-object tests was assessed. In the low-stress environment, 240 pmol of septal urocortin 2 increased anxiety, but lower doses (0.48, 4.8, and 48 pmol) did not have consistent effects. However, in the high-stress condition, 48 pmol of septal urocortin 2 significantly increased anxiety compared with control in wild-type but not CRF2 receptor knock-out mice in the light-dark box. Septal administration of the relatively selective CRF2 antagonist astressin-2B, but not the CRF1-selective antagonist antalarmin, blocked the anxiogenic effects of urocortin 2. Urocortin 2 infusion into the medial septum or lateral ventricle did not affect anxiety measures. These results indicate that the effect of septal CRF2 receptor activation on anxiety is dependent on stress level.
Peptide ligand binding properties of the corticotropin-releasing factor (CRF) type 2 receptor: pharmacology of endogenously expressed receptors, G-protein-coupling sensitivity and determinants of CRF2 receptor selectivity.[Pubmed:15652653]
Peptides. 2005 Mar;26(3):457-70.
The CRF2 receptor is involved in stress responses, cardiovascular function and gastric motility. Endogenous agonists (urocortin (UCN) 2, UCN 3) and synthetic antagonists (astressin2-B, antisauvagine-30) are selective for CRF2 over the CRF1 receptor. Peptide ligand binding properties of the CRF2 receptor require further investigation, including ligand affinity for endogenously expressed receptors, the effect of receptor-G-protein coupling on ligand affinity, and the molecular basis of ligand selectivity. Ligand affinity for rat CRF(2a) in olfactory bulb and CRF(2b) in A7r5 cells was similar to that for the cloned human CRF(2a) receptor (within three-fold), except for oCRF (9.4- and 5.4-fold higher affinity in olfactory bulb and A7r5 cells, respectively). Receptor-G-protein uncoupling reduced agonist affinity only 1.2- to 6.5-fold (compared with 92-1300-fold for the CRF1 receptor). Ligand selectivity mechanisms were investigated using chimeric CRF2/CRF1 receptors. The juxtamembrane receptor domain determined selectivity of antisauvagine-30, the N-terminal-extracellular domain contributed to selectivity of UCN 3, and both domains contributed to selectivity of UCN 2 and astressin2-B. Therefore ligands differ in the contribution of receptor domains to their selectivity, and CRF2-selective antagonists bind the juxtamembrane domain. These findings will be important for identifying the CRF2 receptor in tissues and for developing ligands targeting the receptor, both of which will be useful in identifying the emerging physiological functions of the CRF2 receptor.
Potent and long-acting corticotropin releasing factor (CRF) receptor 2 selective peptide competitive antagonists.[Pubmed:12361401]
J Med Chem. 2002 Oct 10;45(21):4737-47.
We present evidence that members of the corticotropin releasing factor (CRF) family assume distinct structures when interacting with the CRF(1) and CRF(2) receptors. Predictive methods, physicochemical measurements, and structure-activity relationship studies have suggested that CRF, its family members, and competitive antagonists such as astressin [cyclo(30-33)[DPhe(12),Nle(21),Glu(30),Lys(33),Nle(38)]hCRF((12-41))] assume an alpha-helical conformation when interacting with their receptors. We had shown that alpha-helical CRF((9-41)) and sauvagine showed some selectivity for CRF receptors other than that responsible for ACTH secretion(1) and later for CRF2.(2) More recently, we suggested the possibility of a helix-turn-helix motif around a turn encompassing residues 30-33(3) that would confer high affinity for both CRF(1) and CRF(2)(2,4) in agonists and antagonists of all members of the CRF family.(3) On the other hand, the substitutions that conferred ca. 100-fold CRF(2) selectivity to the antagonist antisauvagine-30 [[DPhe(11),His(12)]sauvagine((11-40))] did not confer such property to the corresponding N-terminally extended agonists. We find here that a Glu(32)-Lys(35) side chain to side chain covalent lactam constraint in hCRF and the corresponding Glu(31)-Lys(34) side chain to side chain covalent lactam constraint in sauvagine yield potent ligands that are selective for CRF(2). Additionally, we introduced deletions and substitutions known to increase duration of action to yield antagonists such as cyclo(31-34)[DPhe(11),His(12),C(alpha)MeLeu(13,39),Nle(17),Glu(31),Lys(34)]Ac-sau vagine((8-40)) (astressin(2)-B) with CRF(2) selectivities greater than 100-fold. CRF receptor autoradiography was performed in rat tissue known to express CRF(2) and CRF(1) in order to confirm that astressin(2)-B could indeed bind to established CRF(2) but not CRF(1) receptor-expressing tissues. Extended duration of action of astressin(2)-B vs that of antisauvagine-30 is demonstrated in the CRF(2)-mediated animal model whereby the inhibition of gastric emptying of a solid meal in mice by urocortin administered intraperitoneally at time zero is antagonized by the administration of astressin(2)-B but not by antisauvagine-30 at times -3 and -6 h while both peptides are effective when given 10 min before urocortin.


