(S)-AMPACAS# 83643-88-3 |
- I-BET-762
Catalog No.:BCC4474
CAS No.:1260907-17-2
- Bromodomain Inhibitor, (+)-JQ1
Catalog No.:BCC1132
CAS No.:1268524-70-4
- I-BET151 (GSK1210151A)
Catalog No.:BCC4476
CAS No.:1300031-49-5
- GSK1324726A
Catalog No.:BCC4038
CAS No.:1300031-52-0
- PFI-1 (PF-6405761)
Catalog No.:BCC2225
CAS No.:1403764-72-6
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 83643-88-3 | SDF | Download SDF |
| PubChem ID | 158397 | Appearance | Powder |
| Formula | C7H10N2O4 | M.Wt | 186.17 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble to 100 mM in water | ||
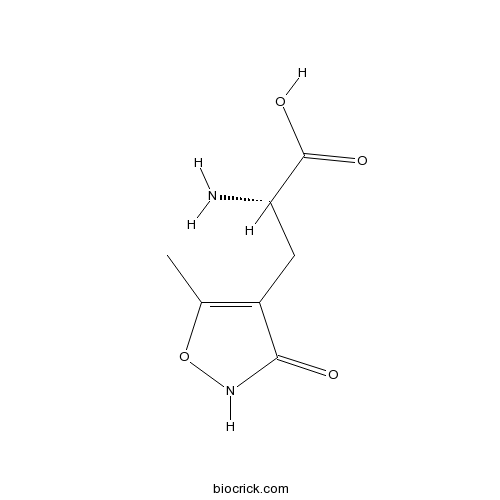
| Chemical Name | (2S)-2-amino-3-(5-methyl-3-oxo-1,2-oxazol-4-yl)propanoic acid | ||
| SMILES | CC1=C(C(=O)NO1)CC(C(=O)O)N | ||
| Standard InChIKey | UUDAMDVQRQNNHZ-YFKPBYRVSA-N | ||
| Standard InChI | InChI=1S/C7H10N2O4/c1-3-4(6(10)9-13-3)2-5(8)7(11)12/h5H,2,8H2,1H3,(H,9,10)(H,11,12)/t5-/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Active enantiomer of AMPA (EC50 = 3.5 μM). Also available as part of the AMPA Receptor. NPEC-caged-(S)-AMPA, inactive enantiomer (R)-AMPA and racemate (RS)-AMPA are also available. |

(S)-AMPA Dilution Calculator

(S)-AMPA Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 5.3714 mL | 26.8572 mL | 53.7143 mL | 107.4287 mL | 134.2859 mL |
| 5 mM | 1.0743 mL | 5.3714 mL | 10.7429 mL | 21.4857 mL | 26.8572 mL |
| 10 mM | 0.5371 mL | 2.6857 mL | 5.3714 mL | 10.7429 mL | 13.4286 mL |
| 50 mM | 0.1074 mL | 0.5371 mL | 1.0743 mL | 2.1486 mL | 2.6857 mL |
| 100 mM | 0.0537 mL | 0.2686 mL | 0.5371 mL | 1.0743 mL | 1.3429 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Cyclosporin H
Catalog No.:BCC6448
CAS No.:83602-39-5
- Cropodine
Catalog No.:BCN2073
CAS No.:83601-85-8
- Regorafenib hydrochloride
Catalog No.:BCC1883
CAS No.:835621-07-3
- 2,2-Bis(3-amino-4-hydroxyphenyl)hexafluoropropane
Catalog No.:BCC8490
CAS No.:83558-87-6
- Anisofolin A
Catalog No.:BCN4377
CAS No.:83529-71-9
- Leucosceptoside A
Catalog No.:BCN7457
CAS No.:83529-62-8
- 4-[4-(3-Hydroxyphenyl)-3-(4-methylphenyl)-6-oxo-1,4-dihydropyrrolo[3,4-d]pyrazol-5-yl]benzoic acid
Catalog No.:BCC6341
CAS No.:834903-43-4
- Grantaline
Catalog No.:BCN2083
CAS No.:83482-61-5
- Ginsenoside Rd2
Catalog No.:BCN8279
CAS No.:83480-64-2
- Voglibose
Catalog No.:BCC4750
CAS No.:83480-29-9
- Mifamurtide
Catalog No.:BCC5241
CAS No.:83461-56-7
- Ginsenoside Ra1
Catalog No.:BCN8392
CAS No.:83459-41-0
- CGRP (rat)
Catalog No.:BCC5712
CAS No.:83651-90-5
- RHC 80267
Catalog No.:BCC8083
CAS No.:83654-05-1
- (R)-AMPA
Catalog No.:BCC6582
CAS No.:83654-13-1
- Dihydrosesamin
Catalog No.:BCN6616
CAS No.:83708-70-7
- Ilexside I
Catalog No.:BCN3244
CAS No.:83725-19-3
- Pomolic acid 28-O-beta-D-glucopyranosyl ester
Catalog No.:BCN1334
CAS No.:83725-24-0
- 3alpha-Acetoxy-20(29)-lupene-23,28-dioic acid
Catalog No.:BCN7508
CAS No.:83725-41-1
- Salvinorin A
Catalog No.:BCC5875
CAS No.:83729-01-5
- WH-4-023
Catalog No.:BCC8051
CAS No.:837422-57-8
- YM 244769
Catalog No.:BCC6222
CAS No.:837424-39-2
- Fmoc-Arg(Tos)-OH
Catalog No.:BCC3076
CAS No.:83792-47-6
- Fmoc-Ser(Bzl)-OH
Catalog No.:BCC3542
CAS No.:83792-48-7
Studies on an (S)-2-amino-3-(3-hydroxy-5-methyl-4-isoxazolyl)propionic acid (AMPA) receptor antagonist IKM-159: asymmetric synthesis, neuroactivity, and structural characterization.[Pubmed:23432124]
J Med Chem. 2013 Mar 28;56(6):2283-93.
IKM-159 was developed and identified as a member of a new class of heterotricyclic glutamate analogues that act as AMPA receptor-selective antagonists. However, it was not known which enantiomer of IKM-159 was responsible for its pharmacological activities. Here, we report in vivo and in vitro neuronal activities of both enantiomers of IKM-159 prepared by enantioselective asymmetric synthesis. By employment of (R)-2-amino-2-(4-methoxyphenyl)ethanol as a chiral auxiliary, (2R)-IKM-159 and the (2S)-counterpart were successfully synthesized in 0.70% and 1.5% yields, respectively, over a total of 18 steps. Both behavioral and electrophysiological assays showed that the biological activity observed for the racemic mixture was reproduced only with (2R)-IKM-159, whereas the (2S)-counterpart was inactive in both assays. Racemic IKM-159 was crystallized with the ligand-binding domain of GluA2, and the structure revealed a complex containing (2R)-IKM-159 at the glutamate binding site. (2R)-IKM-159 locks the GluA2 in an open form, consistent with a pharmacological action as competitive antagonist of AMPA receptors.
AMPA receptor upregulation in the nucleus accumbens shell of cocaine-sensitized rats depends upon S-nitrosylation of stargazin.[Pubmed:24035918]
Neuropharmacology. 2014 Feb;77:28-38.
Behavioral sensitization to cocaine is associated with increased AMPA receptor (AMPAR) surface expression in the nucleus accumbens (NAc). This upregulation is withdrawal-dependent, as it is not detected on withdrawal day (WD) 1, but is observed on WD7-21. Its underlying mechanisms have not been clearly established. Nitric oxide (NO) regulates AMPAR trafficking in the brain by S-nitrosylation of the AMPAR auxiliary subunit, stargazin, leading to increased AMPAR surface expression. Our goal was to determine if stargazin S-nitrosylation contributes to AMPAR upregulation during sensitization. First, we measured stargazin S-nitrosylation in NAc core and shell subregions on WD14 after 8 daily injections of saline or 15 mg/kg cocaine. Stargazin S-nitrosylation was markedly increased in NAc shell but not core. To determine if this is associated with AMPAR upregulation, rats received 8 cocaine or saline injections followed by twice-daily treatments with vehicle or the nitric oxide synthase inhibitor l-NAME (50 mg/kg) on WD1-6, the time when AMPAR upregulation is developing in cocaine-exposed rats. Cocaine/vehicle rats showed elevated stargazin and GluA1 surface expression on WD7 compared to saline/vehicle rats; the GluA1 increase was more robust in core, while stargazin increased more robustly in shell. These effects of cocaine were attenuated in shell but not core when cocaine injections were followed by l-NAME treatment on WD1-6. Together, these results indicate that elevated S-nitrosylation of stargazin contributes to AMPAR upregulation during sensitization selectively in the NAc shell. It is possible that AMPAR upregulation in core involves a different TARP, gamma4, which also upregulates in the NAc of sensitized rats.
S-palmitoylation regulates AMPA receptors trafficking and function: a novel insight into synaptic regulation and therapeutics.[Pubmed:26579419]
Acta Pharm Sin B. 2015 Jan;5(1):1-7.
Glutamate acting on AMPA-type ionotropic glutamate receptor (AMPAR) mediates the majority of fast excitatory synaptic transmission in the mammalian central nervous system. Dynamic regulation of AMPAR by post-translational modifications is one of the key elements that allow the nervous system to adapt to environment stimulations. S-palmitoylation, an important lipid modification by post-translational addition of a long-chain fatty acid to a cysteine residue, regulates AMPA receptor trafficking, which dynamically affects multiple fundamental brain functions, such as learning and memory. In vivo, S-palmitoylation is controlled by palmitoyl acyl transferases and palmitoyl thioesterases. In this review, we highlight advances in the mechanisms for dynamic AMPA receptors palmitoylation, and discuss how palmitoylation affects AMPA receptors function at synapses in recent years. Pharmacological regulation of S-palmitoylation may serve as a novel therapeutic strategy for neurobiological diseases.
Tweaking Subtype Selectivity and Agonist Efficacy at (S)-2-Amino-3-(3-hydroxy-5-methyl-isoxazol-4-yl)propionic acid (AMPA) Receptors in a Small Series of BnTetAMPA Analogues.[Pubmed:26862980]
J Med Chem. 2016 Mar 10;59(5):2244-54.
A series of analogues of the (S)-2-Amino-3-(3-hydroxy-5-methyl-isoxazol-4-yl)propionic acid (AMPA) receptor agonist BnTetAMPA (5b) were synthesized and characterized pharmacologically in radioligand binding assays at native and cloned AMPA receptors and functionally by two-electrode voltage clamp electrophysiology at the four homomeric AMPA receptors expressed in Xenopus laevis oocytes. The analogues 6 and 7 exhibit very different pharmacological profiles with binding affinity preference for the subtypes GluA1 and GluA3, respectively. X-ray crystal structures of three ligands (6, 7, and 8) in complex with the agonist binding domain (ABD) of GluA2 show that they induce full domain closure despite their low agonist efficacies. Trp767 in GluA2 ABD could be an important determinant for partial agonism of this compound series at AMPA receptors, since agonist efficacy also correlated with the location of the Trp767 side chain.
Heteroaryl analogues of AMPA. 2. Synthesis, absolute stereochemistry, photochemistry, and structure-activity relationships.[Pubmed:9651156]
J Med Chem. 1998 Jul 2;41(14):2513-23.
We have previously shown that (S)-2-amino-3-(3-hydroxy-5-phenyl-4-isoxazolyl)propionic acid [(S)-APPA, 2] is a weak agonist at (RS)-2-amino-3-(3-hydroxy-5-methyl-4-isoxazolyl)propionic acid (AMPA) receptors, specifically activated by (S)-AMPA (1), whereas (S)-2-amino-3-[3-hydroxy-5-(2-pyridyl)-4-isoxazolyl]propionic acid [(S)-2-Py-AMPA, 5] and (RS)-2-amino-3-[3-hydroxy-5-(2-thiazolyl)-4-isoxazolyl]propionic acid (4) are potent AMPA agonists. On the other hand, (R)-APPA (3) and (R)-2-Py-AMPA (6) have been shown to be weak AMPA antagonists. We now report the synthesis of 2-Py-AMPA (7a) and the isomeric compounds 3-Py-AMPA (7b) and 4-Py-AMPA (7c) as well as the 7a analogues, (RS)-2-amino-3-[3-hydroxy-5-(6-methyl-2-pyridyl)-4-isoxazolyl]p ropion ic acid (7d) and (RS)-2-amino-3-[3-hydroxy-5-(2-quinolinyl)-4-isoxazolyl]propionic acid (7e). Furthermore, (RS)-2-amino-3-[3-hydroxy-5-(2-furyl)-4-isoxazolyl]propionic acid (2-Fu-AMPA, 7f) and its 5-bromo-2-furyl derivative (7g) were synthesized, and (S)-2-Fu-AMPA (8) and (R)-2-Fu-AMPA (9) were prepared by semipreparative chiral HPLC resolution of 7f. HPLC analyses and circular dichroism spectroscopy indicated the absolute stereochemistry of 8 and 9 to be S and R, respectively. This was confirmed by an X-ray crystallographic analysis of 9.HCl. In receptor binding (IC50 values) and rat cortical wedge electrophysiological (EC50 values) studies, 7c (IC50 = 5.5 +/- 0.6 microM; EC50 = 96 +/- 5 microM) was shown to be markedly weaker than 7a (IC50 = 0.57 +/- 0.16 microM; EC50 = 7.4 +/- 0.2 microM) as an AMPA agonist, whereas 7b,d,e were inactive. The very potent AMPA agonist effect of 7f (IC50 = 0.15 +/- 0.03 microM; EC50 = 1.7 +/- 0. 2 microM) was shown to reside exclusively in 8 (IC50 = 0.11 +/- 0.01 microM; EC50 = 0.71 +/- 0.11 microM), whereas 9 did not interact significantly with AMPA receptors, either as an agonist or as an antagonist. 8 was shown to be photochemically active and is a potential photoaffinity label for the recognition site of the AMPA receptors. Compound 7g turned out to be a very weak AMPA receptor agonist (IC50 = 12 +/- 0.7 microM; EC50 = 160 +/- 15 microM). None of these new compounds showed detectable effects at N-methyl-d-aspartic acid (NMDA) or kainic acid receptors in vitro. The present studies have emphasized that the presence of a heteroatom in the 2-position of the heteroaryl 5-substituent greatly facilitates AMPA receptor agonist activity.
Ibotenic acid analogues. Synthesis, molecular flexibility, and in vitro activity of agonists and antagonists at central glutamic acid receptors.[Pubmed:2859375]
J Med Chem. 1985 May;28(5):668-72.
The syntheses of (RS)-alpha-amino-3-hydroxy-5-tert-butyl-4-isoxazolepropionic acid (9, ATPA), (alpha-RS, beta-RS)-alpha-amino-beta-methyl-3-hydroxy-5-isoxazolepropionic acid (8), (RS)-alpha-amino-3-hydroxy-5-isoxazolebutyric acid (15a), and (RS)-alpha-amino-3-hydroxy-5-isoxazolevaleric acid (15b) are described. The compounds were tested in vitro together with (RS)-alpha-amino-3-hydroxy-5-(bromomethyl)-4-isoxazolepropionic acid (ABPA) as inhibitors of the binding of radioactive-labeled (RS)-alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) to rat brain synaptic membranes. These data were compared with the earlier reported effects of the compounds on single neurons in the feline spinal cord obtained by microelectrophoretic techniques. The three compounds AMPA, ATPA, and ABPA are agonists at the class of receptors assumed to represent a subtype of physiological (S)-glutamic acid (Glu) receptors. Inhibition of [3H]AMPA binding by ATPA was 1 order of magnitude weaker than that of AMPA, in agreement with the relative potency of these compounds in vivo. ABPA proved to be equipotent with AMPA both as an inhibitor of AMPA binding and as a neuronal excitant. The compounds 8, 15a, and 15b have no effect as inhibitors of AMPA binding, in agreement with in vivo studies that have shown that 8 does not affect the firing of central neurons whereas 15a and 15b are antagonists at NMDA receptors, a subpopulation of excitatory receptors not affected by AMPA. Molecular mechanical calculations on AMPA, ATPA, and ABPA using the program MM2 showed that conformations of AMPA, ABPA, and especially ATPA by rotation of the amino acid side chain have energy barriers. A possible receptor-active conformation is suggested.
Enzymic resolution and binding to rat brain membranes of the glutamic acid agonist alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid.[Pubmed:6133955]
J Med Chem. 1983 Jun;26(6):901-3.
The enantiomers of the glutamic acid central nervous system receptor agonist alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) were prepared via kinetic resolution of the racemic N-acetylated 3-methoxy derivative by reusable, immobilized aminoacylase. L-AMPA was more effective (IC50 = 0.6 microM) than D-AMPA (IC50 = 4.8 microM) in displacing racemic [3H]AMPA from binding sites on rat brain synaptic membranes in agreement with their relative in vivo excitatory potencies.


