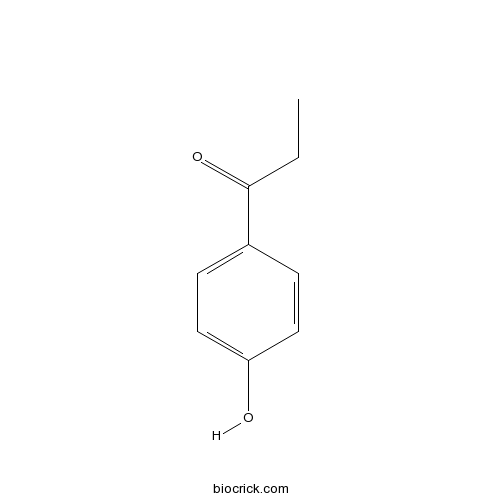
1-(4-Hydroxyphenyl)propan-1-oneCAS# 70-70-2 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 70-70-2 | SDF | Download SDF |
| PubChem ID | 6271 | Appearance | Powder |
| Formula | C9H10O2 | M.Wt | 150.17 |
| Type of Compound | Phenylpropanoids | Storage | Desiccate at -20°C |
| Solubility | DMSO : 100 mg/mL (665.91 mM; Need ultrasonic and warming) | ||
| Chemical Name | 1-(4-hydroxyphenyl)propan-1-one | ||
| SMILES | CCC(=O)C1=CC=C(C=C1)O | ||
| Standard InChIKey | RARSHUDCJQSEFJ-UHFFFAOYSA-N | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Paroxypropione(1-(4-Hydroxyphenyl)propan-1-one) is a manufactured, nonsteroidal estrogen which has been used medically as an antigonadotropin. |
| Targets | Estrogen receptor |
| Structure Identification | Modern Chinese Medicine, 2008,10(11):13-5.Studies on the Chemical Constituents of Alpinia Calanga Willd.[Reference: WebLink]To study the chemical constituents of Alpinia galanga Willd. |

1-(4-Hydroxyphenyl)propan-1-one Dilution Calculator

1-(4-Hydroxyphenyl)propan-1-one Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 6.6591 mL | 33.2956 mL | 66.5912 mL | 133.1824 mL | 166.478 mL |
| 5 mM | 1.3318 mL | 6.6591 mL | 13.3182 mL | 26.6365 mL | 33.2956 mL |
| 10 mM | 0.6659 mL | 3.3296 mL | 6.6591 mL | 13.3182 mL | 16.6478 mL |
| 50 mM | 0.1332 mL | 0.6659 mL | 1.3318 mL | 2.6636 mL | 3.3296 mL |
| 100 mM | 0.0666 mL | 0.333 mL | 0.6659 mL | 1.3318 mL | 1.6648 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- H-Asn-OH
Catalog No.:BCC2875
CAS No.:70-47-3
- L-Glutathione Reduced
Catalog No.:BCC8030
CAS No.:70-18-8
- Triflurdine (Viroptic)
Catalog No.:BCC3873
CAS No.:70-00-8
- Isocostic acid
Catalog No.:BCN4260
CAS No.:69978-82-1
- 20(S),24(R)-Ocotillol
Catalog No.:BCN3891
CAS No.:69926-31-4
- Swertisin
Catalog No.:BCN2762
CAS No.:6991-10-2
- Cyanopindolol hemifumarate
Catalog No.:BCC6880
CAS No.:69906-86-1
- Cinalbicol
Catalog No.:BCN7464
CAS No.:69904-85-4
- Immethridine dihydrobromide
Catalog No.:BCC7328
CAS No.:699020-93-4
- Rhapontisterone B
Catalog No.:BCN2664
CAS No.:698975-64-3
- (+)-Isoajmaline
Catalog No.:BCN3425
CAS No.:6989-79-3
- Evodine
Catalog No.:BCN2630
CAS No.:6989-38-4
- H-Tyr(3-I)-OH
Catalog No.:BCC3265
CAS No.:70-78-0
- Indole-3-carbinol
Catalog No.:BCC5318
CAS No.:700-06-1
- 2-Adamantanol
Catalog No.:BCN8479
CAS No.:700-57-2
- 2-Adamantanone
Catalog No.:BCN8473
CAS No.:700-58-3
- Terazosin HCl
Catalog No.:BCC4354
CAS No.:70024-40-7
- Rivularin
Catalog No.:BCN3189
CAS No.:70028-59-0
- LPYFD-NH2
Catalog No.:BCC6113
CAS No.:700361-48-4
- 4,10-Aromadendranediol
Catalog No.:BCN4261
CAS No.:70051-38-6
- Acronycine
Catalog No.:BCC8114
CAS No.:7008-42-6
- LY2109761
Catalog No.:BCC3806
CAS No.:700874-71-1
- LY2157299
Catalog No.:BCC3709
CAS No.:700874-72-2
- TCN 237 dihydrochloride
Catalog No.:BCC6111
CAS No.:700878-19-9
Phenolic compounds from the branches of Eucalyptus maideni.[Pubmed:22253109]
Chem Biodivers. 2012 Jan;9(1):123-30.
Three new phenolic compounds, eucalmaidin F (1), (3S)-5-guaiacyl-3-hydroxypentanoic acid (2), and 8-beta-C-glucopyranosyl-5,7-dihydroxy-2-isobutylchromone (3), were isolated from the branches of E. maideni, together with 30 known compounds, including four phenylpropanoids, three lignans, four phloroglucinol glucosides, five dihydroflavonoids, seven simple phenolic compounds, six terpenoids, and glycerol. The new structures were established by spectroscopic studies (MS, and 1D- and 2D-NMR), chemical degradation, and modified Mosher's method. Compounds 3, guaiacylglycerol, 3-hydroxy-1-(4-Hydroxyphenyl)propan-1-one, caffeic acid, (2E)-3-(4-hydroxyphenyl)prop-2-enoic acid, (7'S,8R,8'R)-lyoniresinol, (+)-lyoresinol 3alpha-O-alpha-L-rhamnopyranoside, garcimangosone, phlorocetophenone 2'-glucopyranoside, (+)-taxifolin 3alpha-O-alpha-L-rhamnopyranoside, (+)-aromadendrin, (+)-taxifolin, resveratrol, piceatannol, 3,4,5-trihydroxyphenol. Tachiaside, gallic acid, macrocapals A und G, and oleuropeic acid were evaluated for their cytotoxicities against five human cancer cell lines. Resveratrol, piceatannol, gallic acid, and macrocapal G exhibited moderate inhibitory effects on human myeloid heukemia HL-60 cell, with IC(50) values of 22.05, 22.05, 7.75, and 31.93 muM, respectively; and only macrocapal G showed inhibitory effect on hepatocellular carcinoma SMMC-7721 cell, with an IC(50) value of 26.75 muM.
Inactivation of GABA transaminase by 3-chloro-1-(4-hydroxyphenyl)propan-1-one.[Pubmed:19138517]
Bioorg Med Chem Lett. 2009 Feb 1;19(3):731-4.
Previously it was found that 4-hydroxybenzaldehyde is a competitive inhibitor of GABA transaminase. Here 3-chloro-1-(4-Hydroxyphenyl)propan-1-one (9), a 4-hydroxybenzaldehyde analogue, was found to inactivate potently the enzyme in a time-dependent manner. alpha-Ketoglutarate prevented the enzyme from inactivation, suggesting that the inactivation occurs in its active site. Several experiments indicated that the inactivation is irreversible. This study provides a novel strategy for the design of more effective inhibitors.


