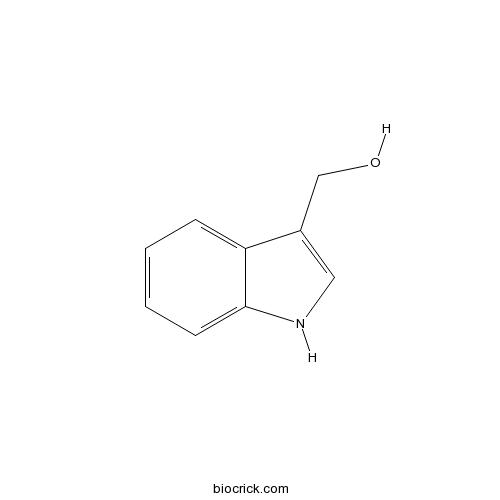
Indole-3-carbinolCAS# 700-06-1 |
- INCB3344
Catalog No.:BCC1648
CAS No.:1262238-11-8
- RS 504393
Catalog No.:BCC1910
CAS No.:300816-15-3
- MK-0812
Catalog No.:BCC1755
CAS No.:624733-88-6
- INCB 3284 dimesylate
Catalog No.:BCC1646
CAS No.:887401-93-6
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 700-06-1 | SDF | Download SDF |
| PubChem ID | 3712 | Appearance | Powder |
| Formula | C9H9NO | M.Wt | 147.18 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | 3-Indolemethanol | ||
| Solubility | DMSO : 150 mg/mL (1019.23 mM; Need ultrasonic and warming) | ||
| Chemical Name | 1H-indol-3-ylmethanol | ||
| SMILES | C1=CC=C2C(=C1)C(=CN2)CO | ||
| Standard InChIKey | IVYPNXXAYMYVSP-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C9H9NO/c11-6-7-5-10-9-4-2-1-3-8(7)9/h1-5,10-11H,6H2 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Indole-3-carbinol Dilution Calculator

Indole-3-carbinol Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 6.7944 mL | 33.972 mL | 67.944 mL | 135.888 mL | 169.86 mL |
| 5 mM | 1.3589 mL | 6.7944 mL | 13.5888 mL | 27.1776 mL | 33.972 mL |
| 10 mM | 0.6794 mL | 3.3972 mL | 6.7944 mL | 13.5888 mL | 16.986 mL |
| 50 mM | 0.1359 mL | 0.6794 mL | 1.3589 mL | 2.7178 mL | 3.3972 mL |
| 100 mM | 0.0679 mL | 0.3397 mL | 0.6794 mL | 1.3589 mL | 1.6986 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Indole-3-carbinol suppresses NF-κB activity and also is an Aryl hydrocarbon receptor (AhR) agonist.
In Vitro:It is found that Indole-3-carbinol (I3C) inhibits the proliferation of THP-1 cells in a dose- and time dependent manner with minimal toxicity over normal monocytes. The AhR target genes (CYP1A1, IL1β) are overexpressed upon Indole-3-carbinol treatment (p<0.05 to p<0.001). The antiproliferative effects of Indole-3-carbinol are in association with programing cell death. Indole-3-carbinol downregulates BCL2 and upregulates FasR in THP-1 cells (p<0.05 to p<0.001). G1 cell cycle arrest is also observed using flow cytometry. G1-acting cell cycle genes (P21, P27 and P53) are overexpressed (p<0.05 to p<0.001), while CDK2 is downregulated upon Indole-3-carbinol treatment (p<0.01 to p<0.001)[1].Indole-3-carbinol suppresses NF-κB activity and stimulates the p53 pathway in pre-B acute lymphoblastic leukemia cells[2].
References:
[1]. Mohammadi S, et al. Indole-3-carbinol induces G1 cell cycle arrest and apoptosis through aryl hydrocarbon receptor in THP-1 monocytic cell line.J Recept Signal Transduct Res. 2017 Oct;37(5):506-514.
[2]. Safa M, et al. Indole-3-carbinol suppresses NF-κB activity and stimulates the p53 pathway in pre-B acute lymphoblastic leukemia cells. Tumour Biol. 2015 May;36(5):3919-30.
- H-Tyr(3-I)-OH
Catalog No.:BCC3265
CAS No.:70-78-0
- 1-(4-Hydroxyphenyl)propan-1-one
Catalog No.:BCN4597
CAS No.:70-70-2
- H-Asn-OH
Catalog No.:BCC2875
CAS No.:70-47-3
- L-Glutathione Reduced
Catalog No.:BCC8030
CAS No.:70-18-8
- Triflurdine (Viroptic)
Catalog No.:BCC3873
CAS No.:70-00-8
- Isocostic acid
Catalog No.:BCN4260
CAS No.:69978-82-1
- 20(S),24(R)-Ocotillol
Catalog No.:BCN3891
CAS No.:69926-31-4
- Swertisin
Catalog No.:BCN2762
CAS No.:6991-10-2
- Cyanopindolol hemifumarate
Catalog No.:BCC6880
CAS No.:69906-86-1
- Cinalbicol
Catalog No.:BCN7464
CAS No.:69904-85-4
- Immethridine dihydrobromide
Catalog No.:BCC7328
CAS No.:699020-93-4
- Rhapontisterone B
Catalog No.:BCN2664
CAS No.:698975-64-3
- 2-Adamantanol
Catalog No.:BCN8479
CAS No.:700-57-2
- 2-Adamantanone
Catalog No.:BCN8473
CAS No.:700-58-3
- Terazosin HCl
Catalog No.:BCC4354
CAS No.:70024-40-7
- Rivularin
Catalog No.:BCN3189
CAS No.:70028-59-0
- LPYFD-NH2
Catalog No.:BCC6113
CAS No.:700361-48-4
- 4,10-Aromadendranediol
Catalog No.:BCN4261
CAS No.:70051-38-6
- Acronycine
Catalog No.:BCC8114
CAS No.:7008-42-6
- LY2109761
Catalog No.:BCC3806
CAS No.:700874-71-1
- LY2157299
Catalog No.:BCC3709
CAS No.:700874-72-2
- TCN 237 dihydrochloride
Catalog No.:BCC6111
CAS No.:700878-19-9
- 5-Heneicosylresorcinol
Catalog No.:BCN7630
CAS No.:70110-59-7
- 5-Tricosyl-1,3-benzenediol
Catalog No.:BCN4262
CAS No.:70110-60-0
Co-treatment with indole-3-carbinol and resveratrol modify porcine CYP1A and CYP3A activities and expression.[Pubmed:28316274]
Xenobiotica. 2018 Mar;48(3):232-240.
1. Humans and animals are commonly exposed to Indole-3-carbinol (I3C) and resveratrol (RES) via food or beverages. Moreover, these compounds have been demonstrated to potentially cause food-drug interactions. However, information about their combined effects is limited. Therefore, we investigated the effects of I3C and RES, both as single compounds and in combination, on cytochrome P450 1A and 3A activity and gene expression. 2. Using porcine microsomes, we demonstrated that RES caused non-competitive inhibition of CYP1A activity and un-competitive inhibition of CYP3A activity. Compared to the effect of single compounds, co-treatment with I3C and RES increased a degree of inhibition of CYP1A activity. 3. In porcine primary hepatocytes, treatment with I3C and RES resulted in induction of CYP1A1, CYP1A2 and CYP3A29 mRNA expression. 4. In conclusion, we demonstrated that both RES and I3C could cause food-drug interactions and that the combined effect could be more potent in doing so.
Preliminary SAR on indole-3-carbinol and related fragments reveals a novel anticancer lead compound against resistant glioblastoma cells.[Pubmed:28256372]
Bioorg Med Chem Lett. 2017 Apr 1;27(7):1561-1565.
The prognosis for glioblastoma patients is, at best, poor, with the median time of survival after diagnosis measured in months. As such, there is much need for the rapid development of potent and novel treatments. Herein, we report our preliminary findings on the SAR of a series of Indole-3-carbinol and related fragments and reveal a potent lead with low micromolar activity against a particularly resistant glioblastoma cell culture, providing a new platform for future development of a new therapy in this area.
Indole-3-carbinol (I3C) analogues are potent small molecule inhibitors of NEDD4-1 ubiquitin ligase activity that disrupt proliferation of human melanoma cells.[Pubmed:27979631]
Biochem Pharmacol. 2017 Mar 1;127:13-27.
The HECT domain-containing E3 ubiquitin ligase NEDD4-1 (Neural precursor cell Expressed Developmentally Down regulated gene 4-1) is frequently overexpressed in human cancers and displays oncogenic-like properties through the ubiquitin-dependent regulation of multiple protein substrates. However, little is known about small molecule enzymatic inhibitors of HECT domain-containing ubiquitin ligases. We now demonstrate that Indole-3-carbinol (I3C), a natural anti-cancer phytochemical derived from cruciferous vegetables such as cabbage and broccoli, represents a new chemical scaffold of small molecule enzymatic inhibitors of NEDD4-1. Using in vitro ubiquitination assays, I3C, its stable synthetic derivative 1-benzyl-I3C and five novel synthetic analogues were shown to directly inhibit NEDD4-1 ubiquitination activity. Compared to I3C, which has an IC50 of 284muM, 1-benzyl-I3C was a significantly more potent NEDD4-1 enzymatic inhibitor with an IC50 of 12.3muM. Compounds 2242 and 2243, the two indolecarbinol analogues with added methyl groups that results in a more nucleophilic benzene ring pi system, further enhanced potency with IC50s of 2.71muM and 7.59muM, respectively. Protein thermal shift assays that assess small ligand binding, in combination with in silico binding simulations with the crystallographic structure of NEDD4-1, showed that each of the indolecarbinol compounds bind to the purified catalytic HECT domain of NEDD4-1. The indolecarbinol compounds inhibited human melanoma cell proliferation in a manner that generally correlated with their effectiveness as NEDD4-1 enzymatic inhibitors. Taken together, we propose that I3C analogues represent a novel set of anti-cancer compounds for treatment of human melanomas and other cancers that express indolecarbinol-sensitive target enzymes.
Enhanced photostability, radical scavenging and antitumor activity of indole-3-carbinol-loaded rose hip oil nanocapsules.[Pubmed:28254296]
Mater Sci Eng C Mater Biol Appl. 2017 May 1;74:279-286.
This study aimed to develop poly(epsilon-caprolactone) nanocapsules loaded with indole-3-cabinol (I3C) using rose hip oil (RHO) or medium chain triglycerides (MCT) as oil core. In vitro radical scavenging activity (DPPH method), hemolysis, and antitumor effects on breast (MCF-7) and glioma (C6) cells were conducted. Preformulation evaluations revealed that RHO is suitable to prepare the nanocapsules considering the log P determination and dissolution/swelling experiments of polymer films. The nanocapsules were prepared and presented adequate physicochemical characteristics as mean size around 250nm, polydispersity index values <0.2, zeta potential negative values and I3C encapsulation efficiency around 42%, without any influence of the oil core (RHO or MCT) on these parameters. However, the photodegradation study demonstrated that RHO nanocapsules showed less degree of I3C degradation in comparison to MCT nanocapsules. The in vitro release profile showed that both nanocapsule suspensions demonstrated an initial burst effect followed by a prolonged I3C release. In addition, the formulations were considered hemocompatibles at 10mug/mL and showed an enhanced radical scavenging activity in comparison to free I3C. Moreover, nanocapsules prepared with RHO increased about two times the antitumor effect of I3C on MCF-7 and C6 cells without significant reduction of astrocyte cell viability. In conclusion, nanocapsule formulations developed in this study might be considered promising for cancer treatment.


