12-Hydroxyjasmonic acidCAS# 140631-27-2 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 140631-27-2 | SDF | Download SDF |
| PubChem ID | 5497122 | Appearance | Powder |
| Formula | C12H18O4 | M.Wt | 226.3 |
| Type of Compound | Miscellaneous | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
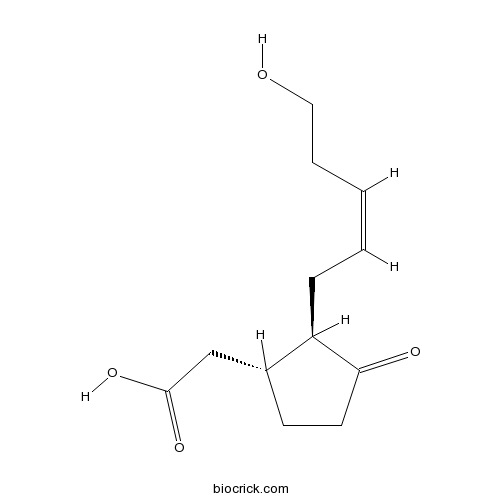
| Chemical Name | 2-[(1R,2R)-2-[(Z)-5-hydroxypent-2-enyl]-3-oxocyclopentyl]acetic acid | ||
| SMILES | C1CC(=O)C(C1CC(=O)O)CC=CCCO | ||
| Standard InChIKey | RZGFUGXQKMEMOO-BSANDHCLSA-N | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | 1. 12-Hydroxyjasmonic acid glucoside is a COI1-JAZ-independent activator of leaf-closing movement in samanea saman. |

12-Hydroxyjasmonic acid Dilution Calculator

12-Hydroxyjasmonic acid Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 4.4189 mL | 22.0946 mL | 44.1891 mL | 88.3783 mL | 110.4728 mL |
| 5 mM | 0.8838 mL | 4.4189 mL | 8.8378 mL | 17.6757 mL | 22.0946 mL |
| 10 mM | 0.4419 mL | 2.2095 mL | 4.4189 mL | 8.8378 mL | 11.0473 mL |
| 50 mM | 0.0884 mL | 0.4419 mL | 0.8838 mL | 1.7676 mL | 2.2095 mL |
| 100 mM | 0.0442 mL | 0.2209 mL | 0.4419 mL | 0.8838 mL | 1.1047 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- 4-Ethylsyringol
Catalog No.:BCN3541
CAS No.:14059-92-8
- Cassipourine
Catalog No.:BCN2154
CAS No.:14051-10-6
- Methyl chanofruticosinate
Catalog No.:BCN6223
CAS No.:14050-92-1
- 1,2-Methylenedioxy-3,10,11-trimethoxynoraporphine
Catalog No.:BCN1573
CAS No.:14050-90-9
- Bacitracin Zinc
Catalog No.:BCC4633
CAS No.:1405-89-6
- Bacitracin
Catalog No.:BCC4632
CAS No.:1405-87-4
- Glycyrrhizic acid
Catalog No.:BCN5941
CAS No.:1405-86-3
- Tylosin phosphate
Catalog No.:BCC5551
CAS No.:1405-53-4
- Gentamycin Sulfate
Catalog No.:BCC1203
CAS No.:1405-41-0
- Capreomycin Sulfate
Catalog No.:BCC4644
CAS No.:1405-37-4
- Neomycin sulfate
Catalog No.:BCC4682
CAS No.:1405-10-3
- Olopatadine HCl
Catalog No.:BCC4545
CAS No.:140462-76-6
- Kadsurenin D
Catalog No.:BCN6603
CAS No.:140669-89-2
- Levatin
Catalog No.:BCN2531
CAS No.:140670-84-4
- 1-Deoxydihydroceramide-1-sulfonic acid
Catalog No.:BCC4964
CAS No.:1407-03-0
- 4-Aza-5androstan-1-ene- 3-one-17carboxylic acid
Catalog No.:BCC8693
CAS No.:140700-63-6
- Scandine Nb-oxide
Catalog No.:BCN7504
CAS No.:140701-69-5
- PF 06465469
Catalog No.:BCC6268
CAS No.:1407966-77-1
- JNK-IN-7
Catalog No.:BCC1672
CAS No.:1408064-71-0
- 1-Hydroxymethyl-beta-carboline glucoside
Catalog No.:BCN7026
CAS No.:1408311-12-5
- ICI 215,001 hydrochloride
Catalog No.:BCC5688
CAS No.:140850-02-8
- Aminopotentidine
Catalog No.:BCC6761
CAS No.:140873-26-3
- 4-Chloro-D-phenylalanine
Catalog No.:BCC2637
CAS No.:14091-08-8
- 6-Hydroxy-5,6-dehydrosugiol
Catalog No.:BCN3127
CAS No.:140923-35-9
The amidohydrolases IAR3 and ILL6 contribute to jasmonoyl-isoleucine hormone turnover and generate 12-hydroxyjasmonic acid upon wounding in Arabidopsis leaves.[Pubmed:24052260]
J Biol Chem. 2013 Nov 1;288(44):31701-14.
Jasmonates (JAs) are a class of signaling compounds that mediate complex developmental and adaptative responses in plants. JAs derive from jasmonic acid (JA) through various enzymatic modifications, including conjugation to amino acids or oxidation, yielding an array of derivatives. The main hormonal signal, jasmonoyl-L-isoleucine (JA-Ile), has been found recently to undergo catabolic inactivation by cytochrome P450-mediated oxidation. We characterize here two amidohydrolases, IAR3 and ILL6, that define a second pathway for JA-Ile turnover during the wound response in Arabidopsis leaves. Biochemical and genetic evidence indicates that these two enzymes cleave the JA-Ile signal, but act also on the 12OH-JA-Ile conjugate. We also show that unexpectedly, the abundant accumulation of tuberonic acid (12OH-JA) after wounding originates partly through a sequential pathway involving (i) conjugation of JA to Ile, (ii) oxidation of the JA-Ile conjugate, and (iii) cleavage under the action of the amidohydrolases. The coordinated actions of oxidative and hydrolytic branches in the jasmonate pathway highlight novel mechanisms of JA-Ile hormone turnover and redefine the dynamic metabolic grid of jasmonate conversion in the wound response.
Polar constituents from the aerial parts of Origanum vulgare L. Ssp. hirtum growing wild in Greece.[Pubmed:16848522]
J Agric Food Chem. 2006 Jul 26;54(15):5388-92.
From the polar extracts of Origanum vulgare L. ssp. hirtum 19 compounds have been isolated. The structures and relative stereochemistry have been elucidated by spectroscopic analysis and determined as apigenin, luteolin, chrysoeriol, diosmetin, quercetin, eriodictyol, cosmoside, vicenin-2, caffeic acid, p-menth-3-ene-1,2-diol 1-O-beta-glucopyranoside, thymoquinol 2-O-beta-glucopyranoside, thymoquinol 5-O-beta-glucopyranoside, thymoquinol 2,5-O-beta-diglucopyranoside, 12-Hydroxyjasmonic acid, 12-Hydroxyjasmonic acid 12-O-beta-glucopyranoside, lithospermic acid B, rosmarinic acid, 10-epi-lithospermic acid, and epi-lithospermic acid B. The three latter products display unusual stereochemistry of the 3,4-hydroxyphenyllactic acid unit(s), which to the authors' best knowledge has never been reported before in similar compounds. Moreover, lithospermic acid B (and its stereoisomers), p-menth-3-ene-1,2-diol 1-O-beta-glucopyranoside, 12-Hydroxyjasmonic acid, and 12-Hydroxyjasmonic acid 12-O-beta-glucopyranoside were isolated for the first time from Origanum species.
12-hydroxyjasmonic acid glucoside is a COI1-JAZ-independent activator of leaf-closing movement in Samanea saman.[Pubmed:21228101]
Plant Physiol. 2011 Mar;155(3):1226-36.
Jasmonates are ubiquitously occurring plant growth regulators with high structural diversity that mediate numerous developmental processes and stress responses. We have recently identified 12-O-beta-D-glucopyranosyljasmonic acid as the bioactive metabolite, leaf-closing factor (LCF), which induced nyctinastic leaf closure of Samanea saman. We demonstrate that leaf closure of isolated Samanea pinnae is induced upon stereospecific recognition of (-)-LCF, but not by its enantiomer, (+)-ent-LCF, and that the nonglucosylated derivative, (-)-12-Hydroxyjasmonic acid also displays weak activity. Similarly, rapid and cell type-specific shrinkage of extensor motor cell protoplasts was selectively initiated upon treatment with (-)-LCF, whereas flexor motor cell protoplasts did not respond. In these bioassays related to leaf movement, all other jasmonates tested were inactive, including jasmonic acid (JA) and the potent derivates JA-isoleucine and coronatine. By contrast, (-)-LCF and (-)-12-Hydroxyjasmonic acid were completely inactive with respect to activation of typical JA responses, such as induction of JA-responsive genes LOX2 and OPCL1 in Arabidopsis (Arabidopsis thaliana) or accumulation of plant volatile organic compounds in S. saman and lima bean (Phaseolus lunatus), generally considered to be mediated by JA-isoleucine in a COI1-dependent fashion. Furthermore, application of selective inhibitors indicated that leaf movement in S. saman is mediated by rapid potassium fluxes initiated by opening of potassium-permeable channels. Collectively, our data point to the existence of at least two separate JA signaling pathways in S. saman and that 12-O-beta-D-glucopyranosyljasmonic acid exerts its leaf-closing activity through a mechanism independent of the COI1-JAZ module.


