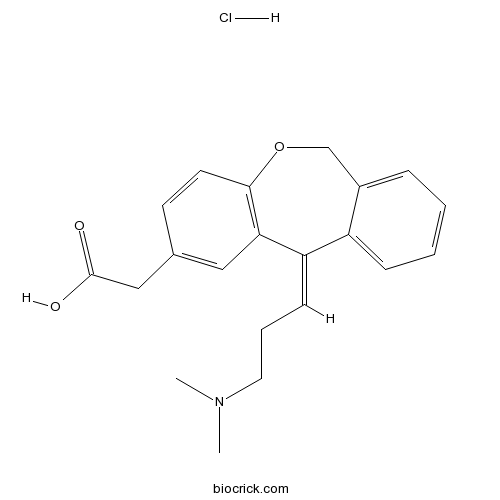
Olopatadine HClHistamine blocker CAS# 140462-76-6 |
- PD 123319 ditrifluoroacetate
Catalog No.:BCC1841
CAS No.:136676-91-0
- Irbesartan
Catalog No.:BCC2560
CAS No.:138402-11-6
- AVE 0991
Catalog No.:BCC4032
CAS No.:304462-19-9
- Tranilast
Catalog No.:BCC2514
CAS No.:53902-12-8
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 140462-76-6 | SDF | Download SDF |
| PubChem ID | 5282402 | Appearance | Powder |
| Formula | C21H24ClNO3 | M.Wt | 373.87 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | DMSO : 50 mg/mL (133.74 mM; Need ultrasonic) H2O : 6.67 mg/mL (17.84 mM; Need ultrasonic) | ||
| Chemical Name | (Z)-11-[3-(Dimethylamino)propyliden | ||
| SMILES | [H+].[Cl-].CN(C)CCC=C/1c2ccccc2COc3ccc(CC(O)=O)cc13 | ||
| Standard InChIKey | HVRLZEKDTUEKQH-NOILCQHBSA-N | ||
| Standard InChI | InChI=1S/C21H23NO3.ClH/c1-22(2)11-5-8-18-17-7-4-3-6-16(17)14-25-20-10-9-15(12-19(18)20)13-21(23)24;/h3-4,6-10,12H,5,11,13-14H2,1-2H3,(H,23,24);1H/b18-8-; | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Histamine H1 receptor antagonist (Ki = 31.6 nM). Inhibits the release of histamine, prostaglandin D2 and tryptase in a concentration-dependent manner. Mast cell stabilizer; inhibits mast cell mediator release. Also suppresses inflammation by inhibition of cytokine production. |

Olopatadine HCl Dilution Calculator

Olopatadine HCl Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.6747 mL | 13.3736 mL | 26.7473 mL | 53.4945 mL | 66.8682 mL |
| 5 mM | 0.5349 mL | 2.6747 mL | 5.3495 mL | 10.6989 mL | 13.3736 mL |
| 10 mM | 0.2675 mL | 1.3374 mL | 2.6747 mL | 5.3495 mL | 6.6868 mL |
| 50 mM | 0.0535 mL | 0.2675 mL | 0.5349 mL | 1.0699 mL | 1.3374 mL |
| 100 mM | 0.0267 mL | 0.1337 mL | 0.2675 mL | 0.5349 mL | 0.6687 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Olopatadine HCl is a histamine blocker used to treat allergic conjunctivitis.Olopatadine is one of the second-generation histamine H1 receptor antagonists that are treated for allergic disorders. Olopatadine significantly inhibited the ear swelling and th
- Heteroclitin B
Catalog No.:BCN3745
CAS No.:140461-47-8
- Heteroclitin C
Catalog No.:BCN3632
CAS No.:140460-42-0
- Ergosterol peroxide glucoside
Catalog No.:BCN6222
CAS No.:140447-22-9
- 11-Hydroxyjasmonic acid
Catalog No.:BCN6221
CAS No.:140447-14-9
- GSK 2830371
Catalog No.:BCC4179
CAS No.:1404456-53-6
- ML 281
Catalog No.:BCC6317
CAS No.:1404437-62-2
- Fmoc-Dap(Dnp)-OH
Catalog No.:BCC2666
CAS No.:140430-54-2
- 7-Methoxycoumarin-4-acetyl-P-L-G-L-β-(2,4-dinitrophenylamino)A-R amide
Catalog No.:BCC1086
CAS No.:140430-53-1
- ON 146040
Catalog No.:BCC8058
CAS No.:1404231-34-0
- CCT244747
Catalog No.:BCC6423
CAS No.:1404095-34-6
- RSVA 405
Catalog No.:BCC8016
CAS No.:140405-36-3
- Vancomycin hydrochloride
Catalog No.:BCC4232
CAS No.:1404-93-9
- Neomycin sulfate
Catalog No.:BCC4682
CAS No.:1405-10-3
- Capreomycin Sulfate
Catalog No.:BCC4644
CAS No.:1405-37-4
- Gentamycin Sulfate
Catalog No.:BCC1203
CAS No.:1405-41-0
- Tylosin phosphate
Catalog No.:BCC5551
CAS No.:1405-53-4
- Glycyrrhizic acid
Catalog No.:BCN5941
CAS No.:1405-86-3
- Bacitracin
Catalog No.:BCC4632
CAS No.:1405-87-4
- Bacitracin Zinc
Catalog No.:BCC4633
CAS No.:1405-89-6
- 1,2-Methylenedioxy-3,10,11-trimethoxynoraporphine
Catalog No.:BCN1573
CAS No.:14050-90-9
- Methyl chanofruticosinate
Catalog No.:BCN6223
CAS No.:14050-92-1
- Cassipourine
Catalog No.:BCN2154
CAS No.:14051-10-6
- 4-Ethylsyringol
Catalog No.:BCN3541
CAS No.:14059-92-8
- 12-Hydroxyjasmonic acid
Catalog No.:BCN6224
CAS No.:140631-27-2
Improved quality of life among seasonal allergic rhinitis patients treated with olopatadine HCl nasal spray 0.4% and olopatadine HCl nasal spray 0.6% compared with vehicle placebo.[Pubmed:16913262]
Allergy Asthma Proc. 2006 May-Jun;27(3):202-7.
Seasonal allergic rhinitis (SAR) exerts a significant adverse impact on health-related quality of life (QoL) and productivity of those who suffer from it. Unfortunately, some therapies for SAR also have a negative impact. Therefore, it is important to scrutinize the influence of new SAR therapies on patients' QoL and ability to function. The purpose of this study was to evaluate the effect of a new nasal antihistamine, olopatadine, on QoL in SAR patients. In a multicenter, randomized, double-blind SAR study comparing olopatadine 0.6 and 0.4% to placebo nasal spray, patients completed the Rhinoconjunctivitis Quality of Life Questionnaire (RQLQ) at baseline and after 2 weeks of treatment. The RQLQ is a validated questionnaire that addresses overall QoL and 7 domains of impairment associated with rhinoconjunctivitis (activities, sleep, non--nose/eye allergy symptoms, practical problems, nasal symptoms, eye symptoms, and emotional impairment). The overall RQLQ mean changes from baseline with olopatadine 0.6% (-1.1 +/- 1.4) and 0.4% (-1.1 +/- 1.3) nasal sprays were superior (p < 0.05) to placebo (-0.8 +/- 1.2). Olopatadine spray 0.6% was superior to placebo in six of the seven RQLQ domains and olopatadine 0.4% was superior to placebo in five RQLQ domains (p < 0.05). The correlation between the olopatadine 0.6% mean total symptom scores and mean RQLQ score was r = 0.66 (p < 0.0001), indicating that the enhancement in QoL derived from olopatadine therapy was significantly associated with symptom reduction. Olopatadine nasal spray is an effective antiallergy medication that significantly improves the QoL of patients suffering from SAR.
Comparison of the effects of ketotifen fumarate 0.025% and olopatadine HCl 0.1% ophthalmic solutions in seasonal allergic conjunctivities: a 30-day, randomized, double-masked, artificial tear substitute-controlled trial.[Pubmed:16291412]
Clin Ther. 2005 Sep;27(9):1392-402.
BACKGROUND: Topical antiallergic agents, such as antihistamines and mast-cell stabilizers, are the main therapeutic options for seasonal allergic conjunctivitis (SAC). Ketotifen fumarate and Olopatadine HCl have dual action that offers a combination of these 2 mechanisms. Although clinical studies comparing the efficacy of these 2 drugs have shown that both were effective in the treatment of SAC, the results were contradictory and did not include the effects of these drugs on inflammatory markers. OBJECTIVES: The aims of this study were to compare the clinical efficacy of topical ketotifen and olopatadine eye drops and to determine the effects of these 2 drugs on the expression of cell adhesion molecules (CAMs) and inflammatory markers in conjunctival surface cells in patients with SAC. METHODS: This 30-day, randomized, double-masked, artificial tear substitute (ATS)-controlled clinical trial was conducted at the Department of Ophthalmology, Karadeniz Technical University, School of Medicine, Trabzon, Turkey. Patients with SAC were included in the study and randomly assigned to 1 of 3 groups: topical ketotifen fumarate 0.025% ophthalmic solution, topical Olopatadine HCl 0.1% ophthalmic solution, or ATS (control group). All drugs were administered 2 drops per eye BID for 30 days. At the beginning of the study (day 0; baseline), on day 15, and on day 30, clinical scores (itching, tearing, redness, eyelid, swelling, and chemosis) and conjunctival impression cytology specimens were obtained. The percentages of cells expressing intercellular adhesion molecule 1, vascular CAM-1, human leukocyte antigen-DR, and beta1-integrin (CD29) from conjunctival impression cytology specimens were determined using flow cytometry. Patients were questioned about adverse events (AEs) at each visit. Ocular discomfort on installation of the drugs was recorded as an AE. RESULTS: Thirty-nine patients (20 men, 19 women; age range, 18-61 years) with SAC were included. Twelve patients received ketotifen; 13, olopatadine; and 14, ATS. In both active-treatment groups, the improvements of clinical scores (tearing and itching) were more pronounced compared with those in the ATS group, although the day-30 difference in tearing score between the olopatadine and ATS groups was not statistically significant. No significant within-group or between-group differences in mean scores for redness, chemosis, or eyelid swelling were found. The expression rates of CAMs and inflammatory markers in conjunctival surface cells were significantly more reduced with ketotifen and olopatadine compared with ATS. However, clinical and flow cytometric parameters were improved with ATS at 15 and 30 days compared with baseline. No AEs were observed during the study period. CONCLUSIONS: In this short-term study in a selected, small study population with SAC, ketotifen and olopatadine diminished the expression of CAMs and inflammatory markers on the conjunctival surface cells effectively. Both active treatments were more efficacious compared with ATS and were well tolerated.
Olopatadine ameliorates rat experimental cutaneous inflammation by improving skin barrier function.[Pubmed:17962722]
Pharmacology. 2008;81(2):118-26.
Olopatadine hydrochloride (olopatadine) is an antiallergic agent with histamine H(1) receptor antagonistic action. We investigated the possible efficacies of olopatadine on the chronic inflammatory dermatitis and the impaired skin barrier functions induced by repeated application of oxazolone in rats. Oxazolone-sensitized rats were challenged with oxazolone applied to the ear every 3 days. Olopatadine was orally administered once daily (1 and 3 mg/kg/day). The effects of the drug were quantified by measurements of ear thickness, levels of cytokines in the lesioned ear and the number of scratching episodes. As parameters of skin barrier function, transepidermal water loss (TEWL) and hyaluronic acid (HA) levels in the lesioned ear were measured. The effect of olopatadine on the production of HA by cultured dermal fibroblasts was also measured. Repeated topical application of oxazolone to rat ears induced local inflammation that was exemplified by swelling. In inflamed ears, the amount of IFN gamma increased at both the protein and mRNA level, but IL-4 levels changed minimally. Olopatadine significantly decreased ear swelling and the number of scratching episodes. The drug also significantly inhibited the increase of IFN gamma and nerve growth factor production in inflamed ears. Olopatadine significantly inhibited the increase in TEWL and the decrease in HA in lesioned ears. Furthermore, the drug stimulated the production of HA by cultured dermal fibroblasts. These results suggest that olopatadine suppressed inflammation and scratching not only by inhibiting cytokine production, but also by repairing skin barrier function.


