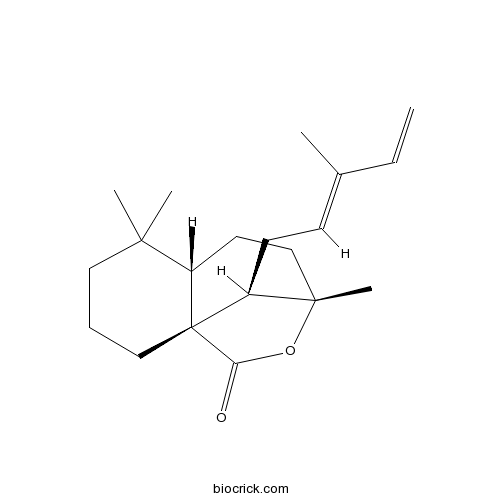
12E,14-Labdadien-20,8beta-olideCAS# 1639257-37-6 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 1639257-37-6 | SDF | Download SDF |
| PubChem ID | 125181777 | Appearance | Powder |
| Formula | C20H30O2 | M.Wt | 302.45 |
| Type of Compound | Diterpenoids | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| SMILES | CC(=CCC1C2(CCC3C1(CCCC3(C)C)C(=O)O2)C)C=C | ||
| Standard InChIKey | SBFYFPUOUKFWPX-MFGREBOPSA-N | ||
| Standard InChI | InChI=1S/C20H30O2/c1-6-14(2)8-9-16-19(5)13-10-15-18(3,4)11-7-12-20(15,16)17(21)22-19/h6,8,15-16H,1,7,9-13H2,2-5H3/b14-8+/t15-,16-,19-,20+/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | 12E,14-Labdadien-20,8beta-olide is a natural product from Isodon yuennanensis. |
| Structure Identification | Angew Chem Int Ed Engl. 2017 May 15;56(21):5844-5848.Bioinspired Asymmetric Synthesis of Hispidanin A.[Pubmed: 28332749 ]
Nat Prod Res. 2015;29(7):628-32.Two new labdane diterpenoids from the rhizomes of Isodon yuennanensis.[Pubmed: 25420949 ]
|

12E,14-Labdadien-20,8beta-olide Dilution Calculator

12E,14-Labdadien-20,8beta-olide Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.3063 mL | 16.5317 mL | 33.0633 mL | 66.1266 mL | 82.6583 mL |
| 5 mM | 0.6613 mL | 3.3063 mL | 6.6127 mL | 13.2253 mL | 16.5317 mL |
| 10 mM | 0.3306 mL | 1.6532 mL | 3.3063 mL | 6.6127 mL | 8.2658 mL |
| 50 mM | 0.0661 mL | 0.3306 mL | 0.6613 mL | 1.3225 mL | 1.6532 mL |
| 100 mM | 0.0331 mL | 0.1653 mL | 0.3306 mL | 0.6613 mL | 0.8266 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- 8(17),12E,14-Labdatrien-20-oic acid
Catalog No.:BCN7396
CAS No.:1639257-36-5
- Androstenediol-3-acetate
Catalog No.:BCC8829
CAS No.:1639-43-6
- Salvianolic acid Y
Catalog No.:BCN8123
CAS No.:1638738-76-7
- 7beta-Hydroxyrutaecarpine
Catalog No.:BCN6500
CAS No.:163815-35-8
- YM 90709
Catalog No.:BCC7149
CAS No.:163769-88-8
- Auristatin F
Catalog No.:BCC5522
CAS No.:163768-50-1
- WIN 64338 hydrochloride
Catalog No.:BCC6914
CAS No.:163727-74-0
- Pazufloxacin mesilate
Catalog No.:BCC9114
CAS No.:163680-77-1
- Kadsulignan L
Catalog No.:BCN3627
CAS No.:163660-06-8
- Evofolin C
Catalog No.:BCN4695
CAS No.:163634-05-7
- Fmoc-D-Trp(Boc)-OH
Catalog No.:BCC3561
CAS No.:163619-04-3
- Kadsulignan N
Catalog No.:BCN3631
CAS No.:163564-58-7
- Kaempferol-3-O-(6''-O-cis-coumaryl)glucoside
Catalog No.:BCN1538
CAS No.:163956-16-9
- Longiferone B
Catalog No.:BCN7378
CAS No.:1639810-67-5
- Clerodenoside A
Catalog No.:BCN1725
CAS No.:164022-75-7
- LY 311727
Catalog No.:BCC7728
CAS No.:164083-84-5
- AM630
Catalog No.:BCC1353
CAS No.:164178-33-0
- Phosphoramidon Disodium Salt
Catalog No.:BCC5484
CAS No.:164204-38-0
- Boc-D-Phe(4-NH2)-OH
Catalog No.:BCC3155
CAS No.:164332-89-2
- Immepip dihydrobromide
Catalog No.:BCC6853
CAS No.:164391-47-3
- KPT-9274
Catalog No.:BCC6461
CAS No.:1643913-93-2
- Aldicarb sulfone
Catalog No.:BCC5476
CAS No.:1646-88-4
- Efinaconazole
Catalog No.:BCC5523
CAS No.:164650-44-6
- Dutasteride
Catalog No.:BCC1097
CAS No.:164656-23-9
Two new labdane diterpenoids from the rhizomes of Isodon yuennanensis.[Pubmed:25420949]
Nat Prod Res. 2015;29(7):628-32.
Two new labdane diterpenoids, s-trans-8(17),12E,14-labdatrien-20-oic acid (1), s-trans-12E,14-Labdadien-20,8beta-olide (2), along with 10 known compounds, hinokiol (3), ursonic acid (4), 2alpha,3alpha-dihydroxyolean-12-en-28-oic acid (5), 2alpha,3beta,23-trihydroxyolean-12-en-28-oic acid (6), ethyl 3-(3,4-dihydroxyphenyl)lactate (7), ethyl rosmarinate (8), (Z,E)-2-(3,4-dihydroxyphenyl)ethenyl caffeic ester (9), tridecanoic acid (10), beta-sitosterol (11) and daucosterol (12), were isolated from the 70% acetone extract of the rhizomes of Isodon yuennanensis. Their structures were elucidated based on the analyses of extensive spectroscopic data and physicochemical properties.
Bioinspired Asymmetric Synthesis of Hispidanin A.[Pubmed:28332749]
Angew Chem Int Ed Engl. 2017 May 15;56(21):5844-5848.
The first enantiospecific synthesis of hispidanin A (4), a dimeric diterpenoid from the rhizomes of Isodon hispida, was achieved with a longest linear sequence of 12 steps in 6.5 % overall yield. A key component is the use of the abundant and naturally occurring diterpenoids (+)-sclareolide and (+)-sclareol as starting materials, which enables the gram-scale preparation of the key intermediates totarane (1) and s-trans-12E,14-Labdadien-20,8beta-olide (2). Subsequently a thermal or an erbium-catalyzed intermolecular Diels-Alder reaction of totarane (1) with labdadienolide (2) provide convergent and rapid access to the natural product hispidanin A (4). The synthetic studies have offered significant impetus for the efficient construction of these architecturally complex natural products.


