3'-Geranyl-3-prenyl-2',4',5,7-tetrahydroxyflavoneCAS# 1334309-44-2 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 1334309-44-2 | SDF | Download SDF |
| PubChem ID | 73554048 | Appearance | Yellow powder |
| Formula | C30H34O6 | M.Wt | 490.6 |
| Type of Compound | Flavonoids | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
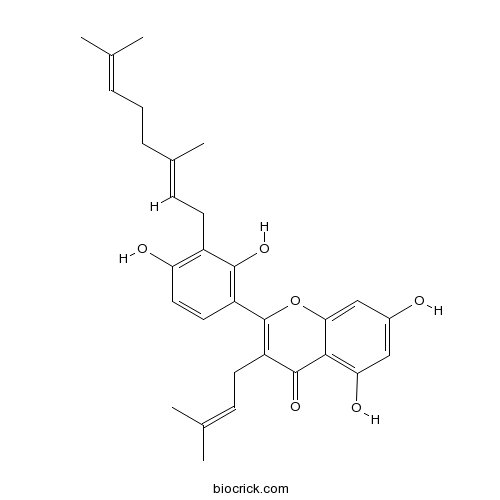
| Chemical Name | 2-[3-[(2E)-3,7-dimethylocta-2,6-dienyl]-2,4-dihydroxyphenyl]-5,7-dihydroxy-3-(3-methylbut-2-enyl)chromen-4-one | ||
| SMILES | CC(=CCCC(=CCC1=C(C=CC(=C1O)C2=C(C(=O)C3=C(C=C(C=C3O2)O)O)CC=C(C)C)O)C)C | ||
| Standard InChIKey | GLWAWFMOTOFEGT-VXLYETTFSA-N | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | 3'-Geranyl-3-prenyl-2',4',5,7-tetrahydroxyflavone is a natural product from Morus alba. |
| In vitro | Cytotoxic prenylated flavonoids from Morus alba.[Pubmed: 20727950 ]Fitoterapia. 2010 Dec;81(8):1224-7.A phytochemical fractionation of the methanol extract of the Morus alba leaves led to the isolation of eleven flavonoids (1-11).
|

3'-Geranyl-3-prenyl-2',4',5,7-tetrahydroxyflavone Dilution Calculator

3'-Geranyl-3-prenyl-2',4',5,7-tetrahydroxyflavone Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.0383 mL | 10.1916 mL | 20.3832 mL | 40.7664 mL | 50.958 mL |
| 5 mM | 0.4077 mL | 2.0383 mL | 4.0766 mL | 8.1533 mL | 10.1916 mL |
| 10 mM | 0.2038 mL | 1.0192 mL | 2.0383 mL | 4.0766 mL | 5.0958 mL |
| 50 mM | 0.0408 mL | 0.2038 mL | 0.4077 mL | 0.8153 mL | 1.0192 mL |
| 100 mM | 0.0204 mL | 0.1019 mL | 0.2038 mL | 0.4077 mL | 0.5096 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- PF 1022A
Catalog No.:BCC8064
CAS No.:133413-70-4
- MG-115
Catalog No.:BCC1237
CAS No.:133407-86-0
- MG-132
Catalog No.:BCC1227
CAS No.:133407-82-6
- (+)-Aflavazole
Catalog No.:BCN7339
CAS No.:133401-09-9
- 3-MPPI
Catalog No.:BCC6705
CAS No.:133399-65-2
- Brandioside
Catalog No.:BCN6770
CAS No.:133393-81-4
- Fmoc-β-Homo-D-Tyr(tBu)-OH
Catalog No.:BCC2620
CAS No.:133373-24-7
- 4-Hydroxy-11,12,13-trinor-5-eudesmen-7-one
Catalog No.:BCN6627
CAS No.:133369-42-3
- Fmoc-His(MMt)-OH
Catalog No.:BCC3503
CAS No.:133367-33-6
- DV 7028 hydrochloride
Catalog No.:BCC6202
CAS No.:133364-62-2
- Daturataturin A
Catalog No.:BCN6179
CAS No.:133360-51-7
- Lydicamycin
Catalog No.:BCN1843
CAS No.:133352-27-9
- Angophorol
Catalog No.:BCN3965
CAS No.:133442-54-3
- Heudelotinone
Catalog No.:BCN6180
CAS No.:133453-58-4
- Iloperidone
Catalog No.:BCC2516
CAS No.:133454-47-4
- Fmoc-Glu(OAll)-OH
Catalog No.:BCC3492
CAS No.:133464-46-7
- TAI-1
Catalog No.:BCC5576
CAS No.:1334921-03-7
- SR 1001
Catalog No.:BCC6309
CAS No.:1335106-03-0
- Exendin-3 (9-39) amide
Catalog No.:BCC7257
CAS No.:133514-43-9
- Apigenin 4'-O-rhamnoside
Catalog No.:BCN6181
CAS No.:133538-77-9
- AG-490
Catalog No.:BCC2193
CAS No.:133550-30-8
- Tyrphostin B44, (-) enantiomer
Catalog No.:BCC6703
CAS No.:133550-32-0
- AG 555
Catalog No.:BCC6721
CAS No.:133550-34-2
- AG 494
Catalog No.:BCC6722
CAS No.:133550-35-3
Assessment of luteolin (3',4',5,7-tetrahydroxyflavone) neuropharmacological activity.[Pubmed:18249450]
Behav Brain Res. 2008 May 16;189(1):75-82.
Since the discovery that certain flavonoids (namely flavones) specifically recognise the central BDZ receptors, several efforts have been made to identify naturally occurring GABA(A) receptor benzodiazepine binding site ligands. Flavonoid derivatives with a flavone-like structure such as apigenin, chrysin and wogonin have been reported for their anxiolytic-like activity in different animal models of anxiety. Luteolin (3',4',5,7-tetrahydroxyflavone) is a widespread flavonoid aglycon that was reported as devoid of specific affinity for benzodiazepine receptor (BDZ-R) binding site, but its psychopharmacological activity is presently unknown. Considering (1) the close structural similarity with other active flavones, (2) the activity of some of its glycosilated derivatives and (3) the complexity of flavonoid effects in the central nervous system, luteolin was submitted to a battery of tests designed to evaluate its possible activity upon the CNS and its ability to interact with the BDZ-receptor binding sites was also analysed. Luteolin apparently has CNS activity with anxiolytic-like effects despite the low affinity for the BDZ-R shown in vitro. Our findings suggest a possible interaction with other neurotransmitter systems but we cannot rule out the possibility that luteolin's metabolites might show a higher affinity for the BDZ-R in vivo, thus eliciting the evident anxiolytic-like effects through a GABAergic mechanism.
Protective effects of kaempferol (3,4',5,7-tetrahydroxyflavone) against amyloid beta peptide (Abeta)-induced neurotoxicity in ICR mice.[Pubmed:20139605]
Biosci Biotechnol Biochem. 2010;74(2):397-401.
To determine the effects of kaempferol, rat pheochromocytoma cells (PC12) and Institute of Cancer Research (ICR) mice were utilized as neuronal models. Using in vitro assays, kaempferol was shown to have protective effects against oxidative stress-induced cytotoxicity in PC12 cells. Administration of kaempferol also significantly reversed amyloid beta peptide (Abeta)-induced impaired performance in a Y-maze test. Taken altogether, the results reported here suggest that further investigation is warranted of the influence of kaempferol on pathways related to Alzheimer's disease.
[Mechanism of growth inhibition effect of 3', 4', 5, 7-tetrahydroxyflavone on A549 cells].[Pubmed:22803372]
Zhongguo Zhong Yao Za Zhi. 2012 May;37(9):1259-64.
OBJECTIVE: To study luteolin-induced non-small cell lung cancer cell line A549 apoptosis and the molecular mechanism for inhibiting its cycle arrest (G2 stage). METHOD: MTT assay showed that luteolin had obvious inhibitory effect on A549 and indicated the half inhibition ratio (IC50). Cell cycle and apoptosis were detected by Hoechst 33258 nuclear staining assay, Annexin V-FITC/PI double staining and flow cytometry. Western blotting assay revealed changes in cycle and apoptosis-related proteins induced by luteolin. Possible molecular mechanism was suggested by Western blotting and immunocytochemistry. RESULT: Luteolin had an obvious growth inhibitory effect on A549 cells, with IC50 of 45.2 micromol x L(-1) at 48 h. Flow cytometry showed A549 cells mainly arrested in G2 stage after being treated by luteolin, with low expressions in cyclin A, p-CDC2 and p-Rb. Hoechst 33258 nuclear staining and Annexin V-FITC/PI double staining showed that the luteolin treatment group showed a significant apoptosis rate than the non-treatment group. Western blotting found luteolin can increase phosphorylation of JNK and decrease that of NF-kappaKB (p65). Immunocytochemistry results revealed luteolin can inhibit TNF-alpha-stimulated p65 from nuclear translocation as a transcription factor and thus promoting cell apoptosis. CONCLUSION: Luteolin can obviously induce apoptosis of human non-small cell lung cancer cell A549 possibly by increasing phosphorylation of JNK to activate mitochondria apoptosis pathway, while inhibiting NF-kappaB from nuclear translocation as a transcription factor.


