5-O-DemethylnobiletinCAS# 2174-59-6 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 2174-59-6 | SDF | Download SDF |
| PubChem ID | 358832 | Appearance | Yellow powder |
| Formula | C20H20O8 | M.Wt | 388.4 |
| Type of Compound | Flavonoids | Storage | Desiccate at -20°C |
| Synonyms | 5-Hydroxy 3',4',6,7,8-pentamethoxyflavone | ||
| Solubility | Soluble in methan | ||
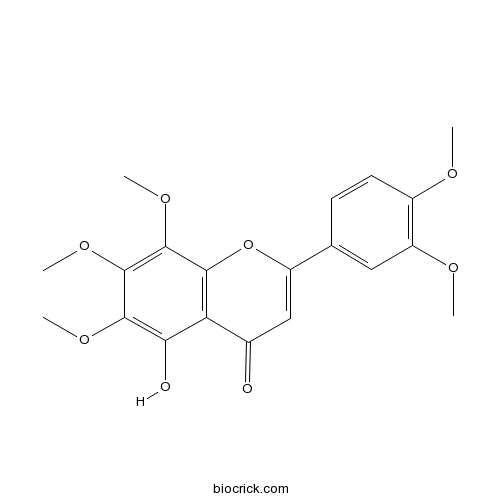
| Chemical Name | 2-(3,4-dimethoxyphenyl)-5-hydroxy-6,7,8-trimethoxychromen-4-one | ||
| SMILES | COC1=C(C=C(C=C1)C2=CC(=O)C3=C(O2)C(=C(C(=C3O)OC)OC)OC)OC | ||
| Standard InChIKey | DOFJNFPSMUCECH-UHFFFAOYSA-N | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | 5-O-Demethylnobiletin has anti-inflammatory activity, it may act through a direct inhibition of 5-LOX, without affecting the expression of COX-2. |
| Targets | COX | LOX |
| In vivo | Anti-inflammatory activity of 5-O-demethylnobiletin, a polymethoxyflavone isolated from Sideritis tragoriganum.[Pubmed: 16491449 ]Planta Med. 2006 Feb;72(2):136-42.
|
| Structure Identification | Food Chem. 2011 Jul 15;127(2):394-403.Chemical composition and biological activity of Citrus jambhiri Lush.[Pubmed: 23140678 ]The fresh peel of Citrus jambhiri was extracted with aqueous methanol and the residue was fractionated using light petroleum, chloroform and ethyl acetate. |

5-O-Demethylnobiletin Dilution Calculator

5-O-Demethylnobiletin Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.5747 mL | 12.8733 mL | 25.7467 mL | 51.4933 mL | 64.3666 mL |
| 5 mM | 0.5149 mL | 2.5747 mL | 5.1493 mL | 10.2987 mL | 12.8733 mL |
| 10 mM | 0.2575 mL | 1.2873 mL | 2.5747 mL | 5.1493 mL | 6.4367 mL |
| 50 mM | 0.0515 mL | 0.2575 mL | 0.5149 mL | 1.0299 mL | 1.2873 mL |
| 100 mM | 0.0257 mL | 0.1287 mL | 0.2575 mL | 0.5149 mL | 0.6437 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Spectinomycin dihydrochloride
Catalog No.:BCC5166
CAS No.:21736-83-4
- Aristoliukine B
Catalog No.:BCN8096
CAS No.:217310-32-2
- PD 173212
Catalog No.:BCC7706
CAS No.:217171-01-2
- Etifoxine
Catalog No.:BCC1560
CAS No.:21715-46-8
- BCX 1470 methanesulfonate
Catalog No.:BCC1414
CAS No.:217099-44-0
- BCX 1470
Catalog No.:BCC1413
CAS No.:217099-43-9
- Hugorosenone
Catalog No.:BCN3776
CAS No.:217096-49-6
- Esomeprazole Magnesium trihydrate
Catalog No.:BCC1559
CAS No.:217087-09-7
- [Pyr1]-Apelin-13
Catalog No.:BCC7358
CAS No.:217082-60-5
- Apelin-17 (human, bovine)
Catalog No.:BCC5959
CAS No.:217082-57-0
- WB 4101 hydrochloride
Catalog No.:BCC6858
CAS No.:2170-58-3
- 15-Epi-Danshenol-A
Catalog No.:BCN3146
CAS No.:216987-13-2
- 5-Methoxyresorcinol
Catalog No.:BCN6904
CAS No.:2174-64-3
- SB 268262
Catalog No.:BCC7916
CAS No.:217438-17-0
- (±)-J 113397
Catalog No.:BCC7423
CAS No.:217461-40-0
- 1-Hydroxy-2-oxopomolic acid
Catalog No.:BCN4931
CAS No.:217466-37-0
- Lucidumol A
Catalog No.:BCN8270
CAS No.:217476-73-8
- L-803,087 trifluoroacetate
Catalog No.:BCC7220
CAS No.:217480-26-7
- L-817,818
Catalog No.:BCC7221
CAS No.:217480-27-8
- Tulathromycin A
Catalog No.:BCC2019
CAS No.:217500-96-4
- Cyclo(Tyr-Val)
Catalog No.:BCN2413
CAS No.:21754-25-6
- Cyclo(Ala-Tyr)
Catalog No.:BCN2412
CAS No.:21754-26-7
- Taxoquinone
Catalog No.:BCN6660
CAS No.:21764-41-0
- BX 471
Catalog No.:BCC6029
CAS No.:217645-70-0
Chemical composition and biological activity of Citrus jambhiri Lush.[Pubmed:23140678]
Food Chem. 2011 Jul 15;127(2):394-403.
The fresh peel of Citrus jambhiri was extracted with aqueous methanol and the residue was fractionated using light petroleum, chloroform and ethyl acetate. The constituents of the extracts were separated by column chromatography employing solvents of different polarity. The chemical structure of the isolated compounds was then identified by MS and NMR. Column chromatography of the petroleum fraction resulted in the isolation of nobiletin, 5-O-Demethylnobiletin, tangeretin, 5-hydroxy-3,6,7,8,3',4'-hexamethoxyflavone, 3,5,6,7,8,3',4'-heptamethoxyflavone, and a mixture of beta-sitosterol and stigmasterol. The chloroform fraction afforded 6-demethoxynobiletin, 5,4'-dihydroxy-6,7,8,3'-tetramethoxyflavone, limonin and nomilin. The flavonoid glycosides naringin, hesperidin and neohesperidin were isolated from the ethyl acetate fraction. The chemical structure of the isolated compounds was established by MS and NMR (APT, COSY, HSQC, HMBC, and NOESY). LC-ESI-MS analysis of the ethyl acetate fraction afforded eight flavonoid glycosides, while the dichloromethane fraction of the defatted seeds contained seven limonoid aglycones. The chloroform fraction exerted the strongest DPPH( *) free radical scavenging activity in comparison to other fractions. The petroleum fraction showed a significant inhibition of lipoxygenase indicating an anti-inflammatory action (IC(50) 29+/-1mug/mL). Some of the isolated polymethoxyflavones exhibited strong cytotoxicity against COS7, HeLa and Caco-2 cell lines.
Anti-inflammatory activity of 5-O-demethylnobiletin, a polymethoxyflavone isolated from Sideritis tragoriganum.[Pubmed:16491449]
Planta Med. 2006 Feb;72(2):136-42.
We have studied the effect of 5- O-demethylnobiletin ( 1) on both the inflammation of mouse ears induced by repeated application of 12- O-tetradecanoylphorbol 13-acetate (TPA) and the acute mouse paw oedemas induced by carrageenan and phospholipase A (2) (PLA (2)), and determined its activity on 5-lipoxygenase (5-LOX) and elastase release/activity. Compound 1 reduced the oedema formation, cell infiltration, and tissue damage in the inflammation induced by TPA in mouse ears, along with the acute oedema induced by carrageenan in mouse paws and the acute PLA (2)-induced oedema in mouse paws. The flavone inhibited leukotriene B (4) formation in rat neutrophils and elastase release in human neutrophils, but did not reduce the expression of cyclooxygenase-2 (COX-2) in murine RAW 264.7 macrophages. These experimental results suggest that 1 may act through a direct inhibition of 5-LOX, without affecting the expression of COX-2.


