Tulathromycin ATriamilide antimicrobial CAS# 217500-96-4 |
- Biapenem
Catalog No.:BCC1071
CAS No.:120410-24-4
- (S)-Tedizolid
Catalog No.:BCC1294
CAS No.:1431699-67-0
- Aprepitant
Catalog No.:BCC1101
CAS No.:170729-80-3
- Doripenem Hydrate
Catalog No.:BCC1160
CAS No.:364622-82-2
- Toltrazuril sulfone
Catalog No.:BCC2008
CAS No.:69004-04-2
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 217500-96-4 | SDF | Download SDF |
| PubChem ID | 9832301 | Appearance | Powder |
| Formula | C41H79N3O12 | M.Wt | 806.08 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | Tulathromycin; CP 472295 | ||
| Solubility | DMSO : 50 mg/mL (62.03 mM; Need ultrasonic) | ||
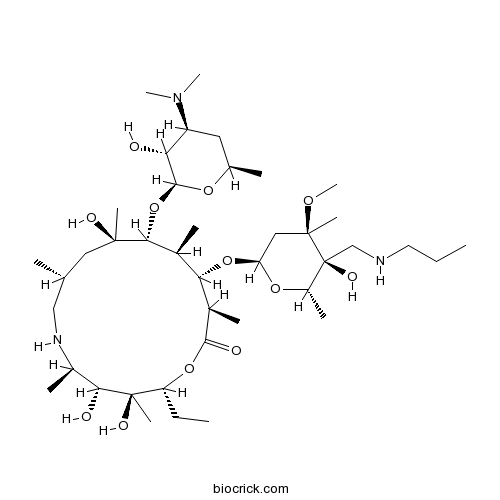
| Chemical Name | (2R,3S,4R,5R,8R,10R,11R,12S,13S,14R)-11-[(2S,3R,4S,6R)-4-(dimethylamino)-3-hydroxy-6-methyloxan-2-yl]oxy-2-ethyl-3,4,10-trihydroxy-13-[(2R,4R,5S,6S)-5-hydroxy-4-methoxy-4,6-dimethyl-5-(propylaminomethyl)oxan-2-yl]oxy-3,5,8,10,12,14-hexamethyl-1-oxa-6-azacyclopentadecan-15-one | ||
| SMILES | CCCNCC1(C(OC(CC1(C)OC)OC2C(C(C(CC(CNC(C(C(C(OC(=O)C2C)CC)(C)O)O)C)C)(C)O)OC3C(C(CC(O3)C)N(C)C)O)C)C)O | ||
| Standard InChIKey | GUARTUJKFNAVIK-QPTWMBCESA-N | ||
| Standard InChI | InChI=1S/C41H79N3O12/c1-15-17-42-22-41(50)28(8)53-31(20-39(41,10)51-14)55-33-25(5)35(56-37-32(45)29(44(12)13)18-24(4)52-37)38(9,48)19-23(3)21-43-27(7)34(46)40(11,49)30(16-2)54-36(47)26(33)6/h23-35,37,42-43,45-46,48-50H,15-22H2,1-14H3/t23-,24-,25+,26-,27-,28+,29+,30-,31+,32-,33+,34-,35-,37+,38-,39-,40-,41+/m1/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Tulathromycin A is a macrolide antibiotic.
IC50 Value: 1 microg/ml (MIC90 for Pasteurella multocida) [2]
Target: Antibacterial
in vitro: Two highly pathogenic strains of M. bovis (with minimum inhibitory concentration values for tulathromycin of 1 and >64 microg/ml) were inoculated into 145 calves. Four days after inoculation, calves with clinical BRD were treated subcutaneously with saline or tulathromycin (2.5 mg/kg). Compared with saline, BRD-related withdrawals, peak rectal temperatures, and lung lesion scores were significantly lower for tulathromycin-treated calves (P < .01). Tulathromycin was highly effective in the treatment of BRD due to M. bovis in calves regardless of the minimum inhibitory concentration of the challenge strain (1 or >64 microg/ml) [1]. The lowest concentrations inhibiting the growth of 90% of isolates (MIC90) for tulathromycin were 2 microg/ml for Mannheimia (Pasteurella) haemolytica, 1 microg/ml for Pasteurella multocida (bovine), and 2 microg/ml for Pasteurella multocida (porcine) and ranged from 0.5 to 4 microg/ml for Histophilus somni (Haemophilus somnus) and from 4 to 16 microg/ml for Actinobacillus pleuropneumoniae [2].
in vivo: Each study randomly allocated 250 calves to receivetulathromycin at 2.5 mg/kg and 250 calves to receive either tilmicosin at 10 mg/kg (Colorado site) or florfenicol at 40 mg/kg (Idaho and Texas sites) on arrival at the feedlot. Calves were housed by treatment group in pens with 50 calves/pen [3]. The treatment groups were physiologic saline (n = 160) given SC at 0.02 ml/kg, tulathromycin (n = 320) given SC at 2.5 mg/kg, and tilmicosin (n = 320) given SC at 10 mg/kg [4].Tulathromycin is a triamilide antimicrobial that has been approved for use in the treatment and prevention of bovine respiratory disease and the treatment of swine respiratory disease.
Toxicity: No adverse events related to tulathromycin were reported [4].
Clinical trial: References: | |||||

Tulathromycin A Dilution Calculator

Tulathromycin A Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 1.2406 mL | 6.2029 mL | 12.4057 mL | 24.8114 mL | 31.0143 mL |
| 5 mM | 0.2481 mL | 1.2406 mL | 2.4811 mL | 4.9623 mL | 6.2029 mL |
| 10 mM | 0.1241 mL | 0.6203 mL | 1.2406 mL | 2.4811 mL | 3.1014 mL |
| 50 mM | 0.0248 mL | 0.1241 mL | 0.2481 mL | 0.4962 mL | 0.6203 mL |
| 100 mM | 0.0124 mL | 0.062 mL | 0.1241 mL | 0.2481 mL | 0.3101 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Tulathromycin A is a macrolide antibiotic [1].Antibiotics are a type of antimicrobial used in the treatment and prevention of bacterial infection.
Two highly pathogenic strains of M. bovis were incubated into 145 calves. Four days later, calves with clinical BRD were treated subcutaneously with tulathromycin or saline (2.5 mg/kg). BRD-related withdrawals, peak rectal temperatures, and lung lesion scores were significantly lower for tulathromycin-treated calves [2]. In the treatment of cattle at high risk of developing bovine respiratory disease, the cure rate treated with tulathromycin (78%) and tilmicosin (65%) was significantly higher than that treated with saline (23.8%). The cure rate treated with tulathromycin (78.4%) was also significantly higher than tilmicosin (64.9%) [3].
The lowest tulathromycin concentrations inhibiting the 90% growth of isolates (MIC90) were 2 μg/ml for Mannheimia (Pasteurella) haemolytica, 1 μg/ml for Pasteurella multocida (bovine), and 2 μg/ml for Pasteurella multocida (porcine) and ranged from 0.5-4 μg/ml for Histophilus somni and from 4-16 μg/ml for Actinobacillus pleuropneumoniae [1]. Tulathromycin has been approved for use in the treatment and prevention of swine and bovine respiratory disease [1].
References:
[1]. Godinho KS, et al. Minimum inhibitory concentrations of tulathromycin against respiratory bacterial pathogens isolated from clinical cases in European cattle and swine and variability arising from changes in in vitro methodology.Vet Ther, 2005, 6(2): 113-121.
[2]. Godinho KS, et al. Efficacy of tulathromycin in the treatment of bovine respiratory disease associated with induced Mycoplasma bovis infections in young dairy calves.Vet Ther, 2005, 6(2): 96-112.
[3]. Kilgore WR, Spensley MS, Sun F, et al. Therapeutic efficacy of tulathromycin, a novel triamilide antimicrobial, against bovine respiratory disease in feeder calves.Vet Ther, 2005, 6(2): 143-153.
- L-817,818
Catalog No.:BCC7221
CAS No.:217480-27-8
- L-803,087 trifluoroacetate
Catalog No.:BCC7220
CAS No.:217480-26-7
- Lucidumol A
Catalog No.:BCN8270
CAS No.:217476-73-8
- 1-Hydroxy-2-oxopomolic acid
Catalog No.:BCN4931
CAS No.:217466-37-0
- (±)-J 113397
Catalog No.:BCC7423
CAS No.:217461-40-0
- SB 268262
Catalog No.:BCC7916
CAS No.:217438-17-0
- 5-Methoxyresorcinol
Catalog No.:BCN6904
CAS No.:2174-64-3
- 5-O-Demethylnobiletin
Catalog No.:BCN2958
CAS No.:2174-59-6
- Spectinomycin dihydrochloride
Catalog No.:BCC5166
CAS No.:21736-83-4
- Aristoliukine B
Catalog No.:BCN8096
CAS No.:217310-32-2
- PD 173212
Catalog No.:BCC7706
CAS No.:217171-01-2
- Etifoxine
Catalog No.:BCC1560
CAS No.:21715-46-8
- Cyclo(Tyr-Val)
Catalog No.:BCN2413
CAS No.:21754-25-6
- Cyclo(Ala-Tyr)
Catalog No.:BCN2412
CAS No.:21754-26-7
- Taxoquinone
Catalog No.:BCN6660
CAS No.:21764-41-0
- BX 471
Catalog No.:BCC6029
CAS No.:217645-70-0
- 4-Cadinen-7-ol
Catalog No.:BCN4932
CAS No.:217650-27-6
- H-Asp(OBzl)-OH
Catalog No.:BCC2885
CAS No.:2177-63-1
- 9,16-Dioxo-10,12,14-octadecatrienoic acid
Catalog No.:BCN1490
CAS No.:217810-46-3
- Curcolonol
Catalog No.:BCN3558
CAS No.:217817-09-9
- Diallyl disulfide
Catalog No.:BCN3840
CAS No.:2179-57-9
- Homomangiferin
Catalog No.:BCN8145
CAS No.:21794-66-1
- Noroxyhydrastinine
Catalog No.:BCN2646
CAS No.:21796-14-5
- Thalifoline
Catalog No.:BCN3301
CAS No.:21796-15-6
Standard PK/PD concepts can be applied to determine a dosage regimen for a macrolide: the case of tulathromycin in the calf.[Pubmed:27501187]
J Vet Pharmacol Ther. 2017 Jan;40(1):16-27.
The pharmacokinetic (PK) profile of tulathromycin, administered to calves subcutaneously at the dosage of 2.5 mg/kg, was established in serum, inflamed (exudate), and noninflamed (transudate) fluids in a tissue cage model. The PK profile of tulathromycin was also established in pneumonic calves. For Mannheimia haemolytica and Pasteurella multocida, tulathromycin minimum inhibitory concentrations (MIC) were approximately 50 times lower in calf serum than in Mueller-Hinton broth. The breakpoint value of the PK/pharmacodynamic (PD) index (AUC(0-24 h) /MIC) to achieve a bactericidal effect was estimated from in vitro time-kill studies to be approximately 24 h for M. haemolytica and P. multocida. A population model was developed from healthy and pneumonic calves and, using Monte Carlo simulations, PK/PD cutoffs required for the development of antimicrobial susceptibility testing (AST) were determined. The population distributions of tulathromycin doses were established by Monte Carlo computation (MCC). The computation predicted a target attainment rate (TAR) for a tulathromycin dosage of 2.5 mg/kg of 66% for M. haemolytica and 87% for P. multocida. The findings indicate that free tulathromycin concentrations in serum suffice to explain the efficacy of single-dose tulathromycin in clinical use, and that a dosage regimen can be computed for tulathromycin using classical PK/PD concepts.
A single laboratory-validated LC-MS method for the analysis of tulathromycin residues in bison and deer sera and selected tissues of white-tailed deer.[Pubmed:27443215]
Drug Test Anal. 2016 May;8(5-6):584-95.
The performance characteristics of a newly developed liquid chromatography-mass spectrometry (LC-MS) method were validated and demonstrated to be fit for purpose in a pharmacokinetic and tissue depletion study of white-tailed deer and bison. Tulathromycin was extracted from bison and deer sera with acetonitrile or trifluoroacetic acid and K2 HPO4 (pH 6.8) buffer solution and cleaned up on a conditioned Bond-Elut cartridge. Tulathromycin, retained on the cartridge; it was eluted with methanol containing 2% formic acid, dried, re-constituted in methanol/1% formic acid, and analyzed by LC-MS. The limit of quantification (LOQ) of the method was 0.6 ng/mL in serum and 0.6 ng/g in tissue with RSDs /=0.99. The validated method was used to quantify the concentration of tulathromycin residues in serum of bison and deer and selected tissue (lung and muscle tissue) samples obtained from 10 healthy, white-tailed deer that were administered the therapeutic dose approved for cattle (i.e., a single 2.5 mg/kg subcutaneous injection of tulathromycin in the neck). The deer were included in a tulathromycin drug depletion study. (c) 2016 Her Majesty the Queen in Right of Canada. Drug Testing and Analysis (c) 2016 John Wiley & Sons, Ltd.
Estimation of tulathromycin depletion in plasma and milk after subcutaneous injection in lactating goats using a nonlinear mixed-effects pharmacokinetic modeling approach.[Pubmed:27863483]
BMC Vet Res. 2016 Nov 18;12(1):258.
BACKGROUND: Extra-label use of tulathromycin in lactating goats is common and may cause violative residues in milk. The objective of this study was to develop a nonlinear mixed-effects pharmacokinetic (NLME-PK) model to estimate tulathromycin depletion in plasma and milk of lactating goats. Eight lactating goats received two subcutaneous injections of 2.5 mg/kg tulathromycin 7 days apart; blood and milk samples were analyzed for concentrations of Tulathromycin And the common fragment of tulathromycin (i.e., the marker residue CP-60,300), respectively, using liquid chromatography mass spectrometry. Based on these new data and related literature data, a NLME-PK compartmental model with first-order absorption and elimination was used to model plasma concentrations and cumulative excreted amount in milk. Monte Carlo simulations with 100 replicates were performed to predict the time when the upper limit of the 95% confidence interval of milk concentrations was below the tolerance. RESULTS: All animals were healthy throughout the study with normal appetite and milk production levels, and with mild-moderate injection-site reactions that diminished by the end of the study. The measured data showed that milk concentrations of the marker residue of tulathromycin were below the limit of detection (LOD = 1.8 ng/ml) 39 days after the second injection. A 2-compartment model with milk as an excretory compartment best described tulathromycin plasma and CP-60,300 milk pharmacokinetic data. The model-predicted data correlated with the measured data very well. The NLME-PK model estimated that tulathromycin plasma concentrations were below LOD (1.2 ng/ml) 43 days after a single injection, and 62 days after the second injection with a 95% confidence. These estimated times are much longer than the current meat withdrawal time recommendation of 18 days for tulathromycin in non-lactating cattle. CONCLUSIONS: The results suggest that twice subcutaneous injections of 2.5 mg/kg Tulathromycin Are a clinically safe extra-label alternative approach for treating pulmonary infections in lactating goats, but a prolonged withdrawal time of at least 39 days after the second injection should be considered to prevent violative residues in milk and any dairy goat being used for meat should have an extended meat withdrawal time.
A large potentiation effect of serum on the in vitro potency of tulathromycin against Mannheimia haemolytica and Pasteurella multocida.[Pubmed:27891615]
J Vet Pharmacol Ther. 2017 Oct;40(5):419-428.
The antimicrobial properties of tulathromycin were investigated for M. haemolytica and P. multocida. Three in vitro indices of antimicrobial activity, minimum inhibitory concentration (MIC), minimum bactericidal concentration (MBC) and time-kill curves, were established for six isolates of each organism. Each index was measured in two growth media: Mueller-Hinton broth (MHB) and calf serum. It was shown that MICs and MBCs were markedly lower in serum than in MHB. MHB:serum ratios for MIC were 47:1 (M. haemolytica) and 53:1 (P. multocida). For both serum and MHB, adjustment of pH led to greater potency at alkaline compared to acid pH. Tulathromycin MIC was influenced by size of inoculum count, being 4.0- to 7.7-fold greater for high compared to low initial counts. It was concluded that for the purpose of determining dosages for therapeutic use, pharmacodynamic data for tulathromycin should be derived in biological fluids such as serum. It is hypothesized that in vitro measurement of MIC in broth, conducted according to internationally recommended standards, may be misleading as a basis for estimating the in vivo potency of tulathromycin.


