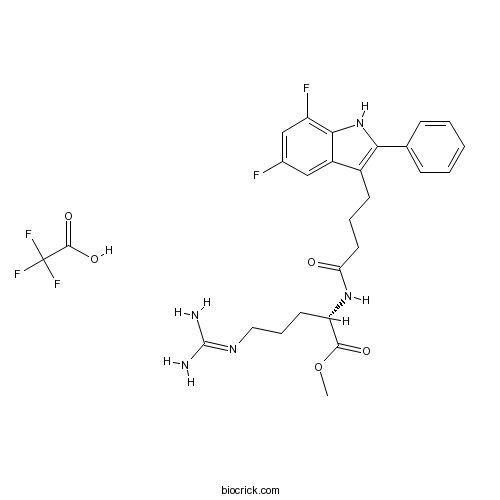
L-803,087 trifluoroacetatePotent and selective sst4 agonist CAS# 217480-26-7 |
- BMS-708163 (Avagacestat)
Catalog No.:BCC2104
CAS No.:1146699-66-2
- DAPT (GSI-IX)
Catalog No.:BCC3618
CAS No.:208255-80-5
- YO-01027 (Dibenzazepine, DBZ)
Catalog No.:BCC2100
CAS No.:209984-56-5
- Semagacestat (LY450139)
Catalog No.:BCC3610
CAS No.:425386-60-3
- Flurizan
Catalog No.:BCC2342
CAS No.:51543-40-9
- E 2012
Catalog No.:BCC1540
CAS No.:870843-42-8
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 217480-26-7 | SDF | Download SDF |
| PubChem ID | 45073436 | Appearance | Powder |
| Formula | C27H30F5N5O5 | M.Wt | 599.56 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble to 100 mM in DMSO and to 100 mM in ethanol | ||
| Chemical Name | methyl (2S)-5-(diaminomethylideneamino)-2-[4-(5,7-difluoro-2-phenyl-1H-indol-3-yl)butanoylamino]pentanoate;2,2,2-trifluoroacetic acid | ||
| SMILES | COC(=O)C(CCCN=C(N)N)NC(=O)CCCC1=C(NC2=C(C=C(C=C12)F)F)C3=CC=CC=C3.C(=O)(C(F)(F)F)O | ||
| Standard InChIKey | IIRYZHRNVKQVGQ-BDQAORGHSA-N | ||
| Standard InChI | InChI=1S/C25H29F2N5O3.C2HF3O2/c1-35-24(34)20(10-6-12-30-25(28)29)31-21(33)11-5-9-17-18-13-16(26)14-19(27)23(18)32-22(17)15-7-3-2-4-8-15;3-2(4,5)1(6)7/h2-4,7-8,13-14,20,32H,5-6,9-12H2,1H3,(H,31,33)(H4,28,29,30);(H,6,7)/t20-;/m0./s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Potent and selective somatostatin sst4 receptor agonist. Ki values are 0.7, 199, 4720, 1280 and 3880 nM for cloned human sst4, sst1, sst2, sst3 and sst5 receptors respectively. Facilitates AMPA-mediated hippocampal synaptic responses in vitro and increases kainate-induced seizures in mice in vivo. |

L-803,087 trifluoroacetate Dilution Calculator

L-803,087 trifluoroacetate Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 1.6679 mL | 8.3394 mL | 16.6789 mL | 33.3578 mL | 41.6972 mL |
| 5 mM | 0.3336 mL | 1.6679 mL | 3.3358 mL | 6.6716 mL | 8.3394 mL |
| 10 mM | 0.1668 mL | 0.8339 mL | 1.6679 mL | 3.3358 mL | 4.1697 mL |
| 50 mM | 0.0334 mL | 0.1668 mL | 0.3336 mL | 0.6672 mL | 0.8339 mL |
| 100 mM | 0.0167 mL | 0.0834 mL | 0.1668 mL | 0.3336 mL | 0.417 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Lucidumol A
Catalog No.:BCN8270
CAS No.:217476-73-8
- 1-Hydroxy-2-oxopomolic acid
Catalog No.:BCN4931
CAS No.:217466-37-0
- (±)-J 113397
Catalog No.:BCC7423
CAS No.:217461-40-0
- SB 268262
Catalog No.:BCC7916
CAS No.:217438-17-0
- 5-Methoxyresorcinol
Catalog No.:BCN6904
CAS No.:2174-64-3
- 5-O-Demethylnobiletin
Catalog No.:BCN2958
CAS No.:2174-59-6
- Spectinomycin dihydrochloride
Catalog No.:BCC5166
CAS No.:21736-83-4
- Aristoliukine B
Catalog No.:BCN8096
CAS No.:217310-32-2
- PD 173212
Catalog No.:BCC7706
CAS No.:217171-01-2
- Etifoxine
Catalog No.:BCC1560
CAS No.:21715-46-8
- BCX 1470 methanesulfonate
Catalog No.:BCC1414
CAS No.:217099-44-0
- BCX 1470
Catalog No.:BCC1413
CAS No.:217099-43-9
- L-817,818
Catalog No.:BCC7221
CAS No.:217480-27-8
- Tulathromycin A
Catalog No.:BCC2019
CAS No.:217500-96-4
- Cyclo(Tyr-Val)
Catalog No.:BCN2413
CAS No.:21754-25-6
- Cyclo(Ala-Tyr)
Catalog No.:BCN2412
CAS No.:21754-26-7
- Taxoquinone
Catalog No.:BCN6660
CAS No.:21764-41-0
- BX 471
Catalog No.:BCC6029
CAS No.:217645-70-0
- 4-Cadinen-7-ol
Catalog No.:BCN4932
CAS No.:217650-27-6
- H-Asp(OBzl)-OH
Catalog No.:BCC2885
CAS No.:2177-63-1
- 9,16-Dioxo-10,12,14-octadecatrienoic acid
Catalog No.:BCN1490
CAS No.:217810-46-3
- Curcolonol
Catalog No.:BCN3558
CAS No.:217817-09-9
- Diallyl disulfide
Catalog No.:BCN3840
CAS No.:2179-57-9
- Homomangiferin
Catalog No.:BCN8145
CAS No.:21794-66-1
Somatostatin receptor subtypes 2 and 4 affect seizure susceptibility and hippocampal excitatory neurotransmission in mice.[Pubmed:12372020]
Eur J Neurosci. 2002 Sep;16(5):843-9.
We have investigated the role of somatostatin receptor subtypes sst2 and sst4 in limbic seizures and glutamate-mediated neurotransmission in mouse hippocampus. As compared to wild-type littermates, homozygous mice lacking sst2 receptors showed a 52% reduction in EEG ictal activity induced by intrahippocampal injection of 30 ng kainic acid (P < 0.05). The number of behavioural tonic-clonic seizures was reduced by 50% (P < 0.01) and the time to onset of seizures was doubled on average (P < 0.05). Seizure-associated neurodegeneration was found in the injected hippocampus (CA1, CA3 and hilar interneurons) and sporadically in the ipsilateral latero-dorsal thalamus. This occurred to a similar extent in wild-type and sst2 knock-out mice. Intrahippocampal injection of three selective sst2 receptor agonists in wild-type mice (Octreotide, BIM 23120 and L-779976, 1.5-6.0 nmol) did not affect kainate seizures while the same compounds significantly reduced seizures in rats. L-803087 (5 nmol), a selective sst4 receptor agonist, doubled seizure activity in wild-type mice on average. Interestingly, this effect was blocked by 3 nmol octreotide. It was determined, in both radioligand binding and cAMP accumulation, that octreotide had no direct agonist or antagonist action at mouse sst4 receptors expressed in CCl39 cells, up to micromolar concentrations. In hippocampal slices from wild-type mice, octreotide (2 micro m) did not modify AMPA-mediated synaptic responses while facilitation occurred with L-803087 (2 micro m). Similarly to what was observed in seizures, the effect of L-803087 was reduced by octreotide. In hippocampal slices from sst2 knock-out mice, both octreotide and L-803087 were ineffective on synaptic responses. Our findings show that, unlike in rats, sst2 receptors in mice do not mediate anticonvulsant effects. Moreover, stimulation of sst4 receptors in the hippocampus of wild-type mice induced excitatory effects which appeared to depend on the presence of sst2 subtypes, suggesting these receptors are functionally coupled.
Identification and characterization of subtype selective somatostatin receptor agonists.[Pubmed:11087999]
J Physiol Paris. 2000 May-Aug;94(3-4):211-5.
High affinity, subtype selective non-peptide agonists of somatostatin receptor subtypes 1-5 were identified in combinatorial libraries constructed based on molecular modeling of known peptide agonists. Simultaneous traditional chemical synthesis yielded an additional series of somatostatin subtype-2 receptor (SSTR2) selective agonists. These compounds have been used to further define the physiological functions of the individual somatostatin receptor subtypes. In vitro experiments demonstrated the role of the SSTR2 in inhibition of glucagon release from mouse pancreatic alpha-cells and the somatostatin subtype-5 receptor (SSTR5) as a mediator of insulin secretion from pancreatic beta-cells. Both SSTR2 and SSTR5 regulated growth hormone release from the rat anterior pituitary gland. In vivo studies performed with SSTR2 receptor selective compounds demonstrated effective inhibition of pulsatile growth hormone release in rats. The SSTR2 selective compounds also lowered plasma glucose levels in normal and diabetic animal models. The availability of high affinity, subtype selective non-peptide agonists for each of the somatostatin receptors provides a direct approach to defining their physiological function both peripherally and in the central nervous system.
Rapid identification of subtype-selective agonists of the somatostatin receptor through combinatorial chemistry.[Pubmed:9784130]
Science. 1998 Oct 23;282(5389):737-40.
Nonpeptide agonists of each of the five somatostatin receptors were identified in combinatorial libraries constructed on the basis of molecular modeling of known peptide agonists. In vitro experiments using these selective compounds demonstrated the role of the somatostatin subtype-2 receptor in inhibition of glucagon release from mouse pancreatic alpha cells and the somatostatin subtype-5 receptor as a mediator of insulin secretion from pancreatic beta cells. Both receptors regulated growth hormone release from the rat anterior pituitary gland. The availability of high-affinity, subtype-selective agonists for each of the somatostatin receptors provides a direct approach to defining their physiological functions.


