8-HydroxypinoresinolCAS# 81426-17-7 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 81426-17-7 | SDF | Download SDF |
| PubChem ID | 3010930 | Appearance | Powder |
| Formula | C20H22O7 | M.Wt | 374.4 |
| Type of Compound | Lignans | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
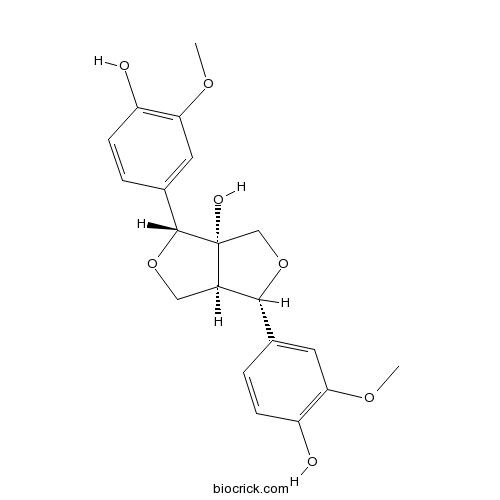
| Chemical Name | (3R,3aS,6S,6aR)-3,6-bis(4-hydroxy-3-methoxyphenyl)-3,4,6,6a-tetrahydro-1H-furo[3,4-c]furan-3a-ol | ||
| SMILES | COC1=C(C=CC(=C1)C2C3COC(C3(CO2)O)C4=CC(=C(C=C4)O)OC)O | ||
| Standard InChIKey | CICMVLOHBZPXIT-WNISUXOKSA-N | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | 1. 8-hydroxypinoresinol has antioxidant activity. 2. 8-hydroxypinoresinol and phillygenin significantly reduce the cell injury by 3-morpholinosydnonimine (SIN-1), a ONOO− generator, may be useful for the therapeutic or preventive applications in treating ONOO−-related diseases. |
| Targets | NO | AChR |

8-Hydroxypinoresinol Dilution Calculator

8-Hydroxypinoresinol Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.6709 mL | 13.3547 mL | 26.7094 mL | 53.4188 mL | 66.7735 mL |
| 5 mM | 0.5342 mL | 2.6709 mL | 5.3419 mL | 10.6838 mL | 13.3547 mL |
| 10 mM | 0.2671 mL | 1.3355 mL | 2.6709 mL | 5.3419 mL | 6.6774 mL |
| 50 mM | 0.0534 mL | 0.2671 mL | 0.5342 mL | 1.0684 mL | 1.3355 mL |
| 100 mM | 0.0267 mL | 0.1335 mL | 0.2671 mL | 0.5342 mL | 0.6677 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- 8-Acetoxypinoresinol
Catalog No.:BCN2161
CAS No.:81426-14-4
- Sanggenone D
Catalog No.:BCN1194
CAS No.:81422-93-7
- Cabergoline
Catalog No.:BCC5276
CAS No.:81409-90-7
- EHNA hydrochloride
Catalog No.:BCC6996
CAS No.:81408-49-3
- Alfuzosin
Catalog No.:BCC4080
CAS No.:81403-80-7
- Alfuzosin HCl
Catalog No.:BCC2494
CAS No.:81403-68-1
- Acetylvalerenolic acid
Catalog No.:BCC8112
CAS No.:81397-67-3
- Fmoc-His(Boc)-OH.CHA
Catalog No.:BCC2595
CAS No.:81379-52-4
- Seglitide
Catalog No.:BCC7191
CAS No.:81377-02-8
- Momordicoside G
Catalog No.:BCN4349
CAS No.:81371-54-2
- Momordicoside K
Catalog No.:BCN3272
CAS No.:81348-84-7
- Momordicoside L
Catalog No.:BCN3274
CAS No.:81348-83-6
- (-)-Pinoresinol
Catalog No.:BCN3254
CAS No.:81446-29-9
- Taxagifine
Catalog No.:BCN6949
CAS No.:81489-69-2
- 1-Hydroxypinoresinol 1-O-glucoside
Catalog No.:BCN7019
CAS No.:81495-71-8
- alpha-Dihydroartemisinin
Catalog No.:BCN2627
CAS No.:81496-81-3
- Nafamostat
Catalog No.:BCC4187
CAS No.:81525-10-2
- Forsythoside B
Catalog No.:BCN1205
CAS No.:81525-13-5
- 2-Pentadecenedioic acid
Catalog No.:BCN3666
CAS No.:81588-35-4
- Neuromedin C (porcine)
Catalog No.:BCC5832
CAS No.:81608-30-2
- Withaperuvin C
Catalog No.:BCN6727
CAS No.:81644-34-0
- Canusesnol A
Catalog No.:BCN4350
CAS No.:816456-90-3
- 3-Dehydro-15-deoxoeucosterol
Catalog No.:BCN4351
CAS No.:81678-46-8
- 18-Beta-hydroxy-3-epi-alpha-yohimbine
Catalog No.:BCN3518
CAS No.:81703-06-2
Cytoprotective effect of lignans from Forsythia suspensa against peroxynitrite-induced LLC-PK1 cell damage.[Pubmed:19367664]
Phytother Res. 2009 Jul;23(7):938-42.
There is mounting evidence that peroxynitrite (ONOO(-)) is closely related to the pathogenesis of various diseases. As a pharmacological strategy aimed at preventing ONOO(-)-mediated toxicity, the protective activity of Forsythia suspensa (Thunb.) Vahl (Oleaceae) against ONOO(-)-induced cellular damage was investigated and its active components identified. After bioactivity-guided fractionation of its methylene chloride fraction, two tetrahydrofurofuran lignans were isolated, namely phillygenin and 8-Hydroxypinoresinol. The protective effects of these lignans against ONOO(-)-induced cell death were evaluated using renal epithelial cell LLC-PK1. Phillygenin and 8-Hydroxypinoresinol significantly reduced the cell injury by 3-morpholinosydnonimine (SIN-1), a ONOO(-) generator. The hydroxy substituents on the phenyl moieties may contribute to the antioxidant activities of these lignans. These results suggest that phillygenin and 8-Hydroxypinoresinol may be useful for the therapeutic or preventive applications in treating ONOO(-)-related diseases.
Lignans from the flowers of Osmanthus fragrans var. aurantiacus and their inhibition effect on NO production.[Pubmed:22210027]
Arch Pharm Res. 2011 Dec;34(12):2029-35.
A new lignan, (7R,7'R,8R,8'R)-8-Hydroxypinoresinol 8-O-beta-D-glucopyranoside 4'-methyl ether (7), was isolated from the flowers of Osmanthus fragrans var. aurantiacus along with six known lignans: (+)-phillygenin (1), phillyrin (2), (-)-phillygenin (3), (-)-epipinoresinol-beta-D-glucoside (4), taxiresinol (5), and (-)-olivil (6). The structure of the new compound was elucidated on the basis of 1D- and 2D-NMR spectroscopic analysis and specific rotation data. The compounds isolated from the flowers of O. fragrans var. aurantiacus were evaluated for inhibitory activities on nitric oxide production in lipopolysaccharide-stimulated macrophage RAW 264.7 cells. (+)-Phillygenin (1), phillyrin (2), and (-)-phillygenin (3) exerted the strongest inhibitory activities on NO production with IC(50) values of 25.5, 18.9, and 25.5 muM, respectively. These compounds may prove beneficial in the development of natural agents for prevention and treatment of inflammatory diseases.
Sesquiterpenoids and lignans from the roots of Valeriana officinalis L.[Pubmed:22006719]
Chem Biodivers. 2011 Oct;8(10):1908-13.
Two new guaiane-type sesquiterpenoids, valerol A (1) and kessyl 3-acetate (2), together with nine known compounds, valeracetate (3), anismol A (4), orientalol C (5), spatulenol (6), 4alpha,10alpha-epoxyaromadendrane (7), (+)-8-Hydroxypinoresinol (8), pinorespiol (9), pinoresinol 4-O-beta-D-glucopyranoside (10), and 8-Hydroxypinoresinol 4'-O-beta-D-glucopyranoside (11) were isolated from the roots of Valeriana officinalis. The structures and relative configurations of 1 and 2 were elucidated on the basis of spectroscopic methods (1D- and 2D-NMR, MS, UV, and IR). These compounds were evaluated for inhibitory activity on acetylcholinesterase (AChE) and enhancing activity on nerve growth factor (NGF)-mediated neurite outgrowth in PC12 cells.


