2-Pentadecenedioic acidCAS# 81588-35-4 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 81588-35-4 | SDF | Download SDF |
| PubChem ID | 12867460 | Appearance | Powder |
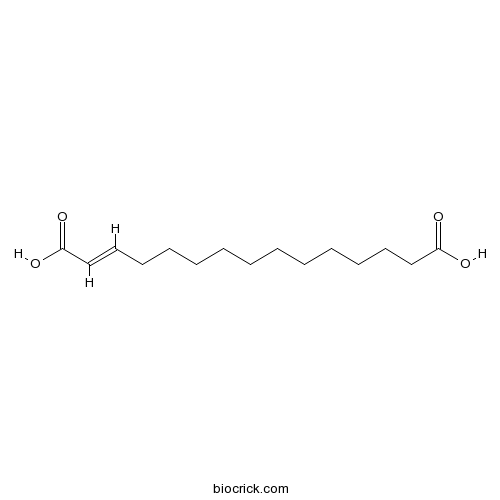
| Formula | C15H26O4 | M.Wt | 270.4 |
| Type of Compound | Miscellaneous | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | (E)-pentadec-2-enedioic acid | ||
| SMILES | C(CCCCCC=CC(=O)O)CCCCCC(=O)O | ||
| Standard InChIKey | JHVMNDBTBXOUQL-ZRDIBKRKSA-N | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

2-Pentadecenedioic acid Dilution Calculator

2-Pentadecenedioic acid Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.6982 mL | 18.4911 mL | 36.9822 mL | 73.9645 mL | 92.4556 mL |
| 5 mM | 0.7396 mL | 3.6982 mL | 7.3964 mL | 14.7929 mL | 18.4911 mL |
| 10 mM | 0.3698 mL | 1.8491 mL | 3.6982 mL | 7.3964 mL | 9.2456 mL |
| 50 mM | 0.074 mL | 0.3698 mL | 0.7396 mL | 1.4793 mL | 1.8491 mL |
| 100 mM | 0.037 mL | 0.1849 mL | 0.3698 mL | 0.7396 mL | 0.9246 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Forsythoside B
Catalog No.:BCN1205
CAS No.:81525-13-5
- Nafamostat
Catalog No.:BCC4187
CAS No.:81525-10-2
- alpha-Dihydroartemisinin
Catalog No.:BCN2627
CAS No.:81496-81-3
- 1-Hydroxypinoresinol 1-O-glucoside
Catalog No.:BCN7019
CAS No.:81495-71-8
- Taxagifine
Catalog No.:BCN6949
CAS No.:81489-69-2
- (-)-Pinoresinol
Catalog No.:BCN3254
CAS No.:81446-29-9
- 8-Hydroxypinoresinol
Catalog No.:BCN3389
CAS No.:81426-17-7
- 8-Acetoxypinoresinol
Catalog No.:BCN2161
CAS No.:81426-14-4
- Sanggenone D
Catalog No.:BCN1194
CAS No.:81422-93-7
- Cabergoline
Catalog No.:BCC5276
CAS No.:81409-90-7
- EHNA hydrochloride
Catalog No.:BCC6996
CAS No.:81408-49-3
- Alfuzosin
Catalog No.:BCC4080
CAS No.:81403-80-7
- Neuromedin C (porcine)
Catalog No.:BCC5832
CAS No.:81608-30-2
- Withaperuvin C
Catalog No.:BCN6727
CAS No.:81644-34-0
- Canusesnol A
Catalog No.:BCN4350
CAS No.:816456-90-3
- 3-Dehydro-15-deoxoeucosterol
Catalog No.:BCN4351
CAS No.:81678-46-8
- 18-Beta-hydroxy-3-epi-alpha-yohimbine
Catalog No.:BCN3518
CAS No.:81703-06-2
- Rehmannioside A
Catalog No.:BCN2885
CAS No.:81720-05-0
- Rehmannioside B
Catalog No.:BCN8468
CAS No.:81720-06-1
- Rehmannioside C
Catalog No.:BCN8183
CAS No.:81720-07-2
- Rhmannioside D
Catalog No.:BCN2362
CAS No.:81720-08-3
- PSI-6130
Catalog No.:BCC1870
CAS No.:817204-33-4
- Bambuterol
Catalog No.:BCC5431
CAS No.:81732-65-2
- Praeruptorin B
Catalog No.:BCN4988
CAS No.:81740-07-0
Effect of calcination temperature of a copper ferrite synthesized by a sol-gel method on its structural characteristics and performance as Fenton catalyst to remove gallic acid from water.[Pubmed:29024859]
J Colloid Interface Sci. 2018 Feb 1;511:193-202.
A copper ferrite synthesized by a sol-gel combustion method was calcined at different temperatures up to 800 degrees C, determining changes in its structural characteristics and magnetic measurements and studying its catalytic performance in gallic acid removal by Fenton reaction. The main objective was to study the effect of the calcination temperature of copper ferrite on its crystalline phase formation and transformation, activity and metal ion leaching. The cubic-to-tetragonal transformation of the spinel occurred via its reaction with the CuO phase, displacing Fe(3+) ions in B (octahedral) sites out of the spinel structure by the following reaction: 2Fe(3+)B+3CuO-->Fe2O3+3Cu(2+)B. The catalysts showed superparamagnetic or substantial superparamagnetic behaviour. At higher calcination temperatures, catalyst activity was lower, and Cu ion leaching was markedly decreased. There was no Fe ion leaching with any catalyst. The as-prepared catalyst showed better catalytic performance than a commercial copper ferrite. Leached Cu ions acted as homogeneous catalysts, and their contribution to the overall removal mechanism was examined. Cu2O present in the as-prepared catalysts made only a small contribution to their activity. Finally, the reutilization of various catalysts was studied by performing different catalytic cycles.
Biochemical, biological and molecular characterization of an L-Amino acid oxidase (LAAO) purified from Bothrops pictus Peruvian snake venom.[Pubmed:29024770]
Toxicon. 2017 Dec 1;139:74-86.
An L-amino acid oxidase from Peruvian Bothrops pictus (Bpic-LAAO) snake venom was purified using a combination of size-exclusion and ion-exchange chromatography. Bpic-LAAO is a homodimeric glycosylated flavoprotein with molecular mass of approximately 65 kDa under reducing conditions and approximately 132 kDa in its native form as analyzed by SDS-PAGE and gel filtration chromatography, respectively. N-terminal amino acid sequencing showed highly conserved residues in a glutamine-rich motif related to binding substrate. The enzyme exhibited optimal activity towards L-Leu at pH 8.5, and like other reported SV-LAAOs, it is stable until 55 degrees C. Kinetic studies showed that the cations Ca(2+), Mg(2+) and Mn(2+) did not alter Bpic-LAAO activity; however, Zn(2+) is an inhibitor. Some reagents such as beta-mercaptoethanol, glutathione and iodoacetate had inhibitory effect on Bpic-LAAO activity, but PMSF, EDTA and glutamic acid did not affect its activity. Regarding the biological activities of Bpic-LAAO, this enzyme induced edema in mice (MED = 7.8 mug), and inhibited human platelet aggregation induced by ADP in a dose-dependent manner and showed antibacterial activity on Gram (+) and Gram (-) bacteria. Bpic-LAAO cDNA of 1494 bp codified a mature protein with 487 amino acid residues comprising a signal peptide of 11 amino acids. Finally, the phylogenetic tree obtained with other sequences of LAAOs, evidenced its similarity to other homologous enzymes, showing two well-established monophyletic groups in Viperidae and Elapidae families. Bpic-LAAO is evolutively close related to LAAOs from B. jararacussu, B. moojeni and B. atrox, and together with the LAAO from B. pauloensis, form a well-defined cluster of the Bothrops genus.
Association between serum uric acid level and renal arteriolar hyalinization in individuals without chronic kidney disease.[Pubmed:29024864]
Atherosclerosis. 2017 Nov;266:121-127.
BACKGROUND AND AIMS: Recent studies have reported an association between serum uric acid (SUA) and renal arteriolar changes in patients with chronic kidney disease (CKD). However, the association in individuals without CKD remains unclear. In this study, we investigated the relationship between SUA and renal arteriolar lesions in individuals without CKD from our living kidney donor cohort. METHODS: Between January 2006 and May 2016, 393 living kidney donors underwent "time-zero" biopsy at Kyushu University Hospital. Patients were divided into sex-specific quartiles of SUA before donation: Q1, Q2, Q3, and Q4 (male: <5.2,5.2-5.8,5.9-6.4, and >/=6.5 mg/dL, female: <3.8,3.8-4.3,4.4-5.0, and >/=5.1 mg/dL). Renal arteriolar hyalinization and wall thickening were assessed using a semiquantitative grading system. Predictive performance was compared between models with and without SUA by calculating the net reclassification improvement (NRI). RESULTS: In total, 158 (40.2%) patients had arteriolar hyalinization, and 148 (37.6%) had wall thickening. High SUA was significantly associated with arteriolar hyalinization in multivariable logistic analysis (odds ratio [OR] per 1-mg/dL increase in SUA, 1.24; 95% confidence interval [CI], 1.00-1.53; p = 0.048. OR for Q4 vs. Q2, 2.22; 95% CI, 1.17-4.21; p = 0.01). We found no association between SUA and wall thickening. When SUA was incorporated into a predictive model with conventional atherosclerotic factors, the NRI was 0.21 (p = 0.04). CONCLUSIONS: High SUA was an independent risk factor for arteriolar hyalinization in individuals without CKD. SUA provided additional predictive value beyond conventional atherosclerotic factors in predicting arteriolar hyalinization.
Dimerization and oxidation of tryptophan in UV-A photolysis sensitized by kynurenic acid.[Pubmed:29024806]
Free Radic Biol Med. 2017 Dec;113:372-384.
Photoinduced generation of radicals in the eye lens may play an important role in the modification of proteins leading to their coloration, aggregation, and insolubilization. The radicals can be formed via the reactions of photoexcited endogenous chromophores of the human lens with lens proteins, in particular with tryptophan residues. In the present work we studied the reactions induced by UV-A (315-400nm) light between kynurenic acid (KNA), an effective photosensitizer present in the human lens, and N-acetyl-L-tryptophan (NTrpH) under aerobic and anaerobic conditions. Our results show that the reaction mechanism strongly depends on the presence of oxygen in solution. Under aerobic conditions, the generation of singlet oxygen is the major channel of the effective NTrpH oxidation. In argon-bubbled solutions, the quenching of triplet KNA by NTrpH results in the formation of KNA(*)(-) and NTrp(*) radicals. Under laser pulse irradiation, when the radical concentration is high, the main pathway of the radical decay is the back electron transfer with the restoration of initial reagents. Other reactions include (i) the radical combination yielding NTrp dimers and (ii) the oxygen atom transfer from KNA(*)(-) to NTrp(*) with the formation of oxidized NTrp species and deoxygenated KNA products. In continuous-wave photolysis, even trace amounts of molecular oxygen are sufficient to oxidize the majority of KNA(*)(-) radicals with the rate constant of (2.0 +/- 0.2) x 10(9)M(-1)s(-1), leading to the restoration of KNA and the formation of superoxide radical O2(*)(-). The latter reacts with NTrp(*) via either the radical combination to form oxidized NTrp (minor pathway), or the electron transfer to restore NTrpH in the ground state (major pathway). As the result, the quantum yields of the starting compound decomposition under continuous-wave anaerobic photolysis are rather low: 1.6% for NTrpH and 0.02% for KNA. The photolysis of KNA with alpha-crystallin yields the same deoxygenated KNA products as the photolysis of KNA with NTrpH, indicating the similarity of the photolysis mechanisms. Thus, inside the eye lens KNA can sensitize both protein photooxidation and protein covalent cross-linking with the minor self-degradation. This may play an important role in the lens protein modifications during the normal aging and cataract development.


