APETx2NaV1.8 and NaV1.2 blocker; also blocks ASIC3 channels CAS# 713544-47-9 |
- LDK378
Catalog No.:BCC3691
CAS No.:1032900-25-6
- LDK378 dihydrochloride
Catalog No.:BCC1694
CAS No.:1380575-43-8
- TAE684 (NVP-TAE684)
Catalog No.:BCC3660
CAS No.:761439-42-3
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 713544-47-9 | SDF | Download SDF |
| PubChem ID | 90488973 | Appearance | Powder |
| Formula | C196H280N54O61S6 | M.Wt | 4561.06 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble to 5 mg/ml in water | ||
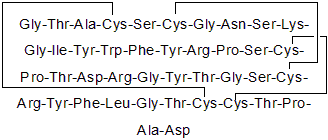
| Sequence | GTACSCGNSKGIYWFYRPSCPTDRGYTGSC (Modifications: Disulfide bridge between 4-37, 6-30, 20-38) | ||
| SMILES | CCC(C)C1C(=O)NC(C(=O)NC(C(=O)NC(C(=O)NC(C(=O)NC(C(=O)N2CCCC2C(=O)NC(C(=O)NC3CSSCC(NC(=O)C4CSSCC(C(=O)NC(C(=O)NC(CSSCC(C(=O)NC(C(=O)NC(C(=O)NC(C(=O)NC(C(=O)NCC(=O)NC(C(=O)N4)C(C)O)CC(C)C)CC5=CC=CC=C5)CC6=CC=C(C=C6)O)CCCNC(=N)N)NC(=O)C(NC(=O)CNC(=O)C(NC(=O)C(NC(=O)CNC(=O)C(NC(=O)C(NC(=O)C(NC(=O)C7CCCN7C3=O)C(C)O)CC(=O)O)CCCNC(=N)N)CC8=CC=C(C=C8)O)C(C)O)CO)C(=O)NCC(=O)NC(C(=O)NC(C(=O)NC(C(=O)NCC(=O)N1)CCCCN)CO)CC(=O)N)CO)NC(=O)C(C)NC(=O)C(C(C)O)NC(=O)CN)C(=O)NC(C(C)O)C(=O)N9CCCC9C(=O)NC(C)C(=O)NC(CC(=O)O)C(=O)O)CO)CCCNC(=N)N)CC1=CC=C(C=C1)O)CC1=CC=CC=C1)CC1=CNC2=CC=CC=C21)CC1=CC=C(C=C1)O | ||
| Standard InChIKey | HEHYILNFEUDIQC-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C196H280N54O61S6/c1-12-94(4)152-186(303)230-125(71-107-48-56-112(263)57-49-107)169(286)229-126(72-108-77-209-114-34-20-19-33-113(108)114)170(287)228-122(67-103-31-17-14-18-32-103)167(284)227-123(69-105-44-52-110(261)53-45-105)165(282)223-118(38-25-61-208-196(204)205)190(307)248-62-26-40-141(248)183(300)235-133(86-254)177(294)241-139-92-317-316-91-138(181(298)247-157(101(11)259)192(309)250-64-28-39-140(250)182(299)215-95(5)158(275)232-129(193(310)311)75-151(273)274)239-180(297)137-90-315-313-88-135(236-159(276)96(6)216-187(304)154(98(8)256)242-144(265)76-198)179(296)234-132(85-253)176(293)237-134(163(280)213-79-146(267)218-127(73-143(199)264)171(288)233-131(84-252)175(292)221-115(35-21-22-58-197)160(277)211-81-148(269)243-152)87-312-314-89-136(178(295)222-117(37-24-60-207-195(202)203)164(281)225-124(70-106-46-54-111(262)55-47-106)168(285)226-121(66-102-29-15-13-16-30-102)166(283)224-119(65-93(2)3)162(279)212-82-149(270)244-155(99(9)257)188(305)240-137)238-174(291)130(83-251)219-147(268)80-214-185(302)153(97(7)255)245-173(290)120(68-104-42-50-109(260)51-43-104)217-145(266)78-210-161(278)116(36-23-59-206-194(200)201)220-172(289)128(74-150(271)272)231-189(306)156(100(10)258)246-184(301)142-41-27-63-249(142)191(139)308/h13-20,29-34,42-57,77,93-101,115-142,152-157,209,251-263H,12,21-28,35-41,58-76,78-92,197-198H2,1-11H3,(H2,199,264)(H,210,278)(H,211,277)(H,212,279)(H,213,280)(H,214,302)(H,215,299)(H,216,304)(H,217,266)(H,218,267)(H,219,268)(H,220,289)(H,221,292)(H,222,295)(H,223,282)(H,224,283)(H,225,281)(H,226,285)(H,227,284)(H,228,287)(H,229,286)(H,230,303)(H,231,306)(H,232,275)(H,233,288)(H,234,296)(H,235,300)(H,236,276)(H,237,293)(H,238,291)(H,239,297)(H,240,305)(H,241,294)(H,242,265)(H,243,269)(H,244,270)(H,245,290)(H,246,301)(H,247,298)(H,271,272)(H,273,274)(H,310,311)(H4,200,201,206)(H4,202,203,207)(H4,204,205,208) | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Acid-sensing ion channel 3 (ASIC3) channel blocker (IC50 values are 63 and 175 nM for homomeric rat and human ASIC3 channels). Also inhibits NaV1.8 and NaV1.2 channels (IC50 values are 55 and 114 nM respectively). Demonstrates analgesic properties against acid-induced and inflammatory pain. |

APETx2 Dilution Calculator

APETx2 Molarity Calculator

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Mcl1-IN-1
Catalog No.:BCC5405
CAS No.:713492-66-1
- Moclobemide (Ro 111163)
Catalog No.:BCC2322
CAS No.:71320-77-9
- 6(1H)-Azulenone, 2,3-dihydro-1,4-dimethyl
Catalog No.:BCN1371
CAS No.:71305-89-0
- (S)-3-Hydroxyphenylglycine
Catalog No.:BCC6605
CAS No.:71301-82-1
- Cronaburmine
Catalog No.:BCN2072
CAS No.:71295-32-4
- Crotananine
Catalog No.:BCN2078
CAS No.:71295-28-8
- 2,5-Bis(5-tert-butyl-2-benzoxazolyl)thiophene
Catalog No.:BCC8503
CAS No.:7128-64-5
- Salaspermic acid
Catalog No.:BCN7139
CAS No.:71247-78-4
- Boc-Ala(2-pyridyl)-OH
Catalog No.:BCC3320
CAS No.:71239-85-5
- Obolactone
Catalog No.:BCN7190
CAS No.:712272-88-3
- Schizandriside
Catalog No.:BCN6999
CAS No.:71222-06-5
- Erythroxytriol P
Catalog No.:BCN4274
CAS No.:7121-99-5
- Jatropholone B
Catalog No.:BCC8192
CAS No.:71386-38-4
- 3-Methyl-GABA
Catalog No.:BCC6629
CAS No.:71424-95-8
- Plinabulin (NPI-2358)
Catalog No.:BCC5094
CAS No.:714272-27-2
- Methylecgonine
Catalog No.:BCN1908
CAS No.:7143-09-1
- Sylvestroside I
Catalog No.:BCN4163
CAS No.:71431-22-6
- TTNPB (Arotinoid Acid)
Catalog No.:BCC4874
CAS No.:71441-28-6
- Z-D-Asp(OtBu)-OH.H2O
Catalog No.:BCC2785
CAS No.:71449-08-6
- H-D-Val-OMe.HCl
Catalog No.:BCC3147
CAS No.:7146-15-8
- Vinorelbine
Catalog No.:BCN2543
CAS No.:71486-22-1
- AC480 (BMS-599626)
Catalog No.:BCC1252
CAS No.:714971-09-2
- Safrolglycol
Catalog No.:BCN4596
CAS No.:7154-01-0
- NSC 319726
Catalog No.:BCC2242
CAS No.:71555-25-4
Functional expression in Escherichia coli of the disulfide-rich sea anemone peptide APETx2, a potent blocker of acid-sensing ion channel 3.[Pubmed:22851929]
Mar Drugs. 2012 Jul;10(7):1605-18.
Acid-sensing ion channels (ASICs) are proton-gated sodium channels present in the central and peripheral nervous system of chordates. ASIC3 is highly expressed in sensory neurons and plays an important role in inflammatory and ischemic pain. Thus, specific inhibitors of ASIC3 have the potential to be developed as novel analgesics. APETx2, isolated from the sea anemone Anthopleura elegantissima, is the most potent and selective inhibitor of ASIC3-containing channels. However, the mechanism of action of APETx2 and the molecular basis for its interaction with ASIC3 is not known. In order to assist in characterizing the ASIC3-APETx2 interaction, we developed an efficient and cost-effective Escherichia coli periplasmic expression system for the production of APETx2. NMR studies on uniformly (13)C/(15)N-labelled APETx2 produced in E. coli showed that the recombinant peptide adopts the native conformation. Recombinant APETx2 is equipotent with synthetic APETx2 at inhibiting ASIC3 channels expressed in Xenopus oocytes. Using this system we mutated Phe15 to Ala, which caused a profound loss of APETx2's activity on ASIC3. These findings suggest that this expression system can be used to produce mutant versions of APETx2 in order to facilitate structure-activity relationship studies.
Understanding the molecular basis of toxin promiscuity: the analgesic sea anemone peptide APETx2 interacts with acid-sensing ion channel 3 and hERG channels via overlapping pharmacophores.[Pubmed:25337890]
J Med Chem. 2014 Nov 13;57(21):9195-203.
The sea anemone peptide APETx2 is a potent and selective blocker of acid-sensing ion channel 3 (ASIC3). APETx2 is analgesic in a variety of rodent pain models, but the lack of knowledge of its pharmacophore and binding site on ASIC3 has impeded development of improved analogues. Here we present a detailed structure-activity relationship study of APETx2. Determination of a high-resolution structure of APETx2 combined with scanning mutagenesis revealed a cluster of aromatic and basic residues that mediate its interaction with ASIC3. We show that APETx2 also inhibits the off-target hERG channel by reducing the maximal current amplitude and shifting the voltage dependence of activation to more positive potentials. Electrophysiological screening of selected APETx2 mutants revealed partial overlap between the surfaces on APETx2 that mediate its interaction with ASIC3 and hERG. Characterization of the molecular basis of these interactions is an important first step toward the rational design of more selective APETx2 analogues.
The selective ASIC3 inhibitor APETx2 alleviates gastric mucosal lesion in the rat.[Pubmed:25073401]
Pharmazie. 2014 Jul;69(7):542-6.
This study aimed to assess the in vivo efficacy of acid sensing ion channel 3 (ASIC3) inhibitor APETx2 to alleviate acute gastric mucosal lesion (AGML) in a rat model. Thirty-six male Wistar rats were divided randomly into three groups: control group, water immersion restraint stress (WIRS) group, and APETx2 treatment group (n = 12). AGML was induced by WIRS for 6 h, and 25 microg/kg APETx2 was injected intraperitoneally before the onset of stress. Intragastric pH, ulcer index (UI) and gastric histopathological changes were measured, ASIC3 expression in thoracic dorsal root ganglia (DRG) neurons was examined by immunohistochemistry, PCR and Western blot analysis. Compared with control group, WIRS group showed obvious gastric injury with increased UI score, decreased intragastric pH and increased ASIC3 expression in DRG neurons (p < 0.05). APETx2 treatment before WIRS significantly alleviated gastric mucosal injury, decreased UI score, decreased gastric acidity and reduced ASIC3 expression in thoracic DRG neurons (p < 0.05). In conclusion, ASIC3 expression in DRG neurons projecting to the stomach is positively correlated with gastric mucosal lesion and acidosis in WIRS model. ASIC3 inhibitor APETx2 could improve gastric acidosis and alleviate AGML.
In silico assessment of interaction of sea anemone toxin APETx2 and acid sensing ion channel 3.[Pubmed:24942880]
Biochem Biophys Res Commun. 2014 Jul 18;450(1):384-9.
Acid sensing ion channels (ASICs) are proton-gated cation channels that are expressed throughout the nervous system and have been implicated in mediating sensory perception of noxious stimuli. Amongst the six ASIC isoforms, ASIC1a, 1b, 2a and 3 form proton-gated homomers, which differ in their activation and inactivation kinetics, expression profiles and pharmacological modulation; protons do not gate ASIC2b and ASIC4. As with many other ion channels, structure-function studies of ASICs have been greatly aided by the discovery of some toxins that act in isoform-specific ways. ASIC3 is predominantly expressed by sensory neurons of the peripheral nervous system where it acts to detect acid as a noxious stimulus and thus plays an important role in nociception. ASIC3 is the only ASIC subunit that is inhibited by the sea anemone (Anthopleura elegantissima)-derived toxin APETx2. However, the molecular mechanism by which APETx2 interacts with ASIC3 remains largely unknown. In this study, we made a homology model of ASIC3 and used extensive protein-protein docking to predict for the first time, the probable sites of APETx2 interaction on ASIC3. Additionally, using computational alanine scanning, we also suggest the 'hot-spots' that are likely to be critical for ASIC3-APETx2 interaction.
Inhibition of voltage-gated Na(+) currents in sensory neurones by the sea anemone toxin APETx2.[Pubmed:21943094]
Br J Pharmacol. 2012 Apr;165(7):2167-77.
BACKGROUND AND PURPOSE: APETx2, a toxin from the sea anemone Anthropleura elegantissima, inhibits acid-sensing ion channel 3 (ASIC3)-containing homo- and heterotrimeric channels with IC(50) values < 100 nM and 0.1-2 microM respectively. ASIC3 channels mediate acute acid-induced and inflammatory pain response and APETx2 has been used as a selective pharmacological tool in animal studies. Toxins from sea anemones also modulate voltage-gated Na(+) channel (Na(v) ) function. Here we tested the effects of APETx2 on Na(v) function in sensory neurones. EXPERIMENTAL APPROACH: Effects of APETx2 on Na(v) function were studied in rat dorsal root ganglion (DRG) neurones by whole-cell patch clamp. KEY RESULTS: APETx2 inhibited the tetrodotoxin (TTX)-resistant Na(v) 1.8 currents of DRG neurones (IC(50) , 2.6 microM). TTX-sensitive currents were less inhibited. The inhibition of Na(v) 1.8 currents was due to a rightward shift in the voltage dependence of activation and a reduction of the maximal macroscopic conductance. The inhibition of Na(v) 1.8 currents by APETx2 was confirmed with cloned channels expressed in Xenopus oocytes. In current-clamp experiments in DRG neurones, the number of action potentials induced by injection of a current ramp was reduced by APETx2. CONCLUSIONS AND IMPLICATIONS: APETx2 inhibited Na(v) 1.8 channels, in addition to ASIC3 channels, at concentrations used in in vivo studies. The limited specificity of this toxin should be taken into account when using APETx2 as a pharmacological tool. Its dual action will be an advantage for the use of APETx2 or its derivatives as analgesic drugs.
A natural point mutation changes both target selectivity and mechanism of action of sea anemone toxins.[Pubmed:22972919]
FASEB J. 2012 Dec;26(12):5141-51.
APETx3, a novel peptide isolated from the sea anemone Anthopleura elegantissima, is a naturally occurring mutant from APETx1, only differing by a Thr to Pro substitution at position 3. APETx1 is believed to be a selective modulator of human ether-a-go-go related gene (hERG) potassium channels with a K(d) of 34 nM. In this study, APETx1, 2, and 3 have been subjected to an electrophysiological screening on a wide range of 24 ion channels expressed in Xenopus laevis oocytes: 10 cloned voltage-gated sodium channels (Na(V) 1.2-Na(V)1.8, the insect channels DmNa(V)1, BgNa(V)1-1a, and the arachnid channel VdNa(V)1) and 14 cloned voltage-gated potassium channels (K(V)1.1-K(V)1.6, K(V)2.1, K(V)3.1, K(V)4.2, K(V)4.3, K(V)7.2, K(V)7.4, hERG, and the insect channel Shaker IR). Surprisingly, the Thr3Pro substitution results in a complete abolishment of APETx3 modulation on hERG channels and provides this toxin the ability to become a potent (EC(50) 276 nM) modulator of voltage-gated sodium channels (Na(V)s) because it slows down the inactivation of mammalian and insect Na(V) channels. Our study also shows that the homologous toxins APETx1 and APETx2 display promiscuous properties since they are also capable of recognizing Na(V) channels with IC(50) values of 31 nM and 114 nM, respectively, causing an inhibition of the sodium conductance without affecting the inactivation. Our results provide new insights in key residues that allow these sea anemone toxins to recognize distinct ion channels with similar potency but with different modulatory effects. Furthermore, we describe for the first time the target promiscuity of a family of sea anemone toxins thus far believed to be highly selective.
Reversal of acid-induced and inflammatory pain by the selective ASIC3 inhibitor, APETx2.[Pubmed:20860671]
Br J Pharmacol. 2010 Oct;161(4):950-60.
BACKGROUND AND PURPOSE: Inflammatory pain is triggered by activation of pathways leading to the release of mediators such as bradykinin, prostaglandins, interleukins, ATP, growth factors and protons that sensitize peripheral nociceptors. The activation of acid-sensitive ion channels (ASICs) may have particular relevance in the development and maintenance of inflammatory pain. ASIC3 is of particular interest due to its restricted tissue distribution in the nociceptive primary afferent fibres and its high sensitivity to protons. EXPERIMENTAL APPROACH: To examine the contribution of ASIC3 to the development and maintenance of muscle pain and inflammatory pain, we studied the in vivo efficacy of a selective ASIC3 inhibitor, APETx2, in rats. KEY RESULTS: Administration of APETx2 into the gastrocnemius muscle prior to the administration of low pH saline prevented the development of mechanical hypersensitivity, whereas APETx2 administration following low-pH saline was ineffective in reversing hypersensitivity. The prevention of mechanical hypersensitivity produced by acid administration was observed whether APETx2 was applied via i.m. or i.t. routes. In the complete Freund's adjuvant (CFA) inflammatory pain model, local administration of APETx2 resulted in a potent and complete reversal of established mechanical hypersensitivity, whereas i.t. application of APETx2 was ineffective. CONCLUSIONS AND IMPLICATIONS: ASIC3 contributed to the development of mechanical hypersensitivity in the acid-induced muscle pain model, whereas ASIC3 contributed to the maintenance of mechanical hypersensitivity in the CFA inflammatory pain model. The contribution of ASIC3 to established hypersensitivity associated with inflammation suggests that this channel may be an effective analgesic target for inflammatory pain states.
Acid-sensing ion channels (ASICs) as pharmacological targets for neurodegenerative diseases.[Pubmed:17945532]
Curr Opin Pharmacol. 2008 Feb;8(1):25-32.
A significant drop of tissue pH or acidosis is a common feature of acute neurological conditions such as ischemic stroke, brain trauma, and epileptic seizures. Acid-sensing ion channels, or ASICs, are proton-gated cation channels widely expressed in peripheral sensory neurons and in the neurons of the central nervous system. Recent studies have demonstrated that activation of these channels by protons plays an important role in a variety of physiological and pathological processes such as nociception, mechanosensation, synaptic plasticity, and acidosis-mediated neuronal injury. This review provides an overview of the recent advance in electrophysiological, pharmacological characterization of ASICs, and their role in neurological diseases. Therapeutic potential of current available ASIC inhibitors is discussed.
A new sea anemone peptide, APETx2, inhibits ASIC3, a major acid-sensitive channel in sensory neurons.[Pubmed:15044953]
EMBO J. 2004 Apr 7;23(7):1516-25.
From a systematic screening of animal venoms, we isolated a new toxin (APETx2) from the sea anemone Anthopleura elegantissima, which inhibits ASIC3 homomeric channels and ASIC3-containing heteromeric channels both in heterologous expression systems and in primary cultures of rat sensory neurons. APETx2 is a 42 amino-acid peptide crosslinked by three disulfide bridges, with a structural organization similar to that of other sea anemone toxins that inhibit voltage-sensitive Na+ and K+ channels. APETx2 reversibly inhibits rat ASIC3 (IC50=63 nM), without any effect on ASIC1a, ASIC1b, and ASIC2a. APETx2 directly inhibits the ASIC3 channel by acting at its external side, and it does not modify the channel unitary conductance. APETx2 also inhibits heteromeric ASIC2b+3 current (IC50=117 nM), while it has less affinity for ASIC1b+3 (IC50=0.9 microM), ASIC1a+3 (IC50=2 microM), and no effect on the ASIC2a+3 current. The ASIC3-like current in primary cultured sensory neurons is partly and reversibly inhibited by APETx2 with an IC50 of 216 nM, probably due to the mixed inhibitions of various co-expressed ASIC3-containing channels.


