2,5-Bis(5-tert-butyl-2-benzoxazolyl)thiopheneCAS# 7128-64-5 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 7128-64-5 | SDF | Download SDF |
| PubChem ID | 292429 | Appearance | Powder |
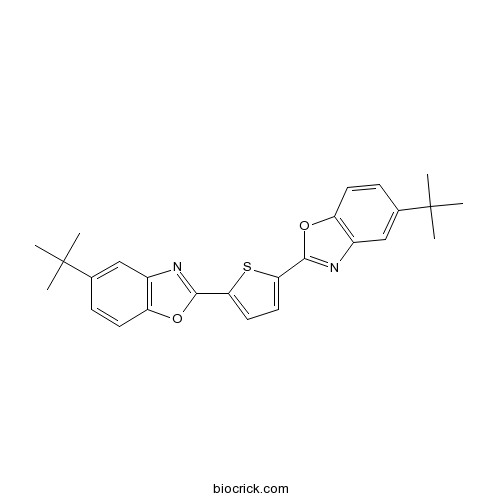
| Formula | C26H26N2O2S | M.Wt | 430.6 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | 5-tert-butyl-2-[5-(5-tert-butyl-1,3-benzoxazol-2-yl)thiophen-2-yl]-1,3-benzoxazole | ||
| SMILES | CC(C)(C)C1=CC2=C(C=C1)OC(=N2)C3=CC=C(S3)C4=NC5=C(O4)C=CC(=C5)C(C)(C)C | ||
| Standard InChIKey | AIXZBGVLNVRQSS-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C26H26N2O2S/c1-25(2,3)15-7-9-19-17(13-15)27-23(29-19)21-11-12-22(31-21)24-28-18-14-16(26(4,5)6)8-10-20(18)30-24/h7-14H,1-6H3 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

2,5-Bis(5-tert-butyl-2-benzoxazolyl)thiophene Dilution Calculator

2,5-Bis(5-tert-butyl-2-benzoxazolyl)thiophene Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.3223 mL | 11.6117 mL | 23.2234 mL | 46.4468 mL | 58.0585 mL |
| 5 mM | 0.4645 mL | 2.3223 mL | 4.6447 mL | 9.2894 mL | 11.6117 mL |
| 10 mM | 0.2322 mL | 1.1612 mL | 2.3223 mL | 4.6447 mL | 5.8059 mL |
| 50 mM | 0.0464 mL | 0.2322 mL | 0.4645 mL | 0.9289 mL | 1.1612 mL |
| 100 mM | 0.0232 mL | 0.1161 mL | 0.2322 mL | 0.4645 mL | 0.5806 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Salaspermic acid
Catalog No.:BCN7139
CAS No.:71247-78-4
- Boc-Ala(2-pyridyl)-OH
Catalog No.:BCC3320
CAS No.:71239-85-5
- Obolactone
Catalog No.:BCN7190
CAS No.:712272-88-3
- Schizandriside
Catalog No.:BCN6999
CAS No.:71222-06-5
- Erythroxytriol P
Catalog No.:BCN4274
CAS No.:7121-99-5
- ML 141
Catalog No.:BCC8092
CAS No.:71203-35-5
- Leukotriene B4
Catalog No.:BCC7322
CAS No.:71160-24-2
- (±)-Bay K 8644
Catalog No.:BCC3918
CAS No.:71145-03-4
- (E)-3-Acetoxy-5-methoxystilbene
Catalog No.:BCN4273
CAS No.:71144-78-0
- Meloxicam (Mobic)
Catalog No.:BCC3808
CAS No.:71125-38-7
- Bucindolol
Catalog No.:BCC7444
CAS No.:71119-11-4
- MRS 2578
Catalog No.:BCC4976
CAS No.:711019-86-2
- Crotananine
Catalog No.:BCN2078
CAS No.:71295-28-8
- Cronaburmine
Catalog No.:BCN2072
CAS No.:71295-32-4
- (S)-3-Hydroxyphenylglycine
Catalog No.:BCC6605
CAS No.:71301-82-1
- 6(1H)-Azulenone, 2,3-dihydro-1,4-dimethyl
Catalog No.:BCN1371
CAS No.:71305-89-0
- Moclobemide (Ro 111163)
Catalog No.:BCC2322
CAS No.:71320-77-9
- Mcl1-IN-1
Catalog No.:BCC5405
CAS No.:713492-66-1
- APETx2
Catalog No.:BCC6294
CAS No.:713544-47-9
- Jatropholone B
Catalog No.:BCC8192
CAS No.:71386-38-4
- 3-Methyl-GABA
Catalog No.:BCC6629
CAS No.:71424-95-8
- Plinabulin (NPI-2358)
Catalog No.:BCC5094
CAS No.:714272-27-2
- Methylecgonine
Catalog No.:BCN1908
CAS No.:7143-09-1
- Sylvestroside I
Catalog No.:BCN4163
CAS No.:71431-22-6
Lattice-Matched Epitaxial Growth of Organic Heterostructures for Integrated Optoelectronic Application.[Pubmed:28233948]
Angew Chem Int Ed Engl. 2017 Mar 20;56(13):3616-3620.
Development of nanowire photonics requires integration of different nanowire components into highly ordered functional heterostructures. Herein, we report a sequential self-assembly of binary molecular components into branched nanowire heterostructures (BNHs) via lattice-matched epitaxial growth, in which the microribbon backbone of 2,5-Bis(5-tert-butyl-2-benzoxazolyl)thiophene (BBOT) functions as blue-emitting microlaser source to pump the nanowire branches of BODIPY. By constructing Au electrodes on both branch sides and measuring the photocurrent in them, we successfully realize the integration of an organic laser and a power meter in a single device. This work provides a new insight into the integration of 1D organic nanostructures into BNHs for realizing organic multifunctional photonic devices.
Antimicrobial metabolites from a marine-derived Actinomycete Streptomyces sp. G278.[Pubmed:29726708]
Nat Prod Res. 2018 May 4:1-8.
Analysis of an antimicrobial extract prepared from culture broth of the marine-derived actinomycete Streptomyces sp. G278 led to the isolation of ten compounds, 1-10. Two compounds, 2,5-Bis(5-tert-butyl-2-benzoxazolyl)thiophene (1), and 3-hydroxyl-2-methylpyridine (2) were isolated from a natural source for the first time. The structures of the isolated compounds were established by their spectral data analysis, including mass spectrometry, 1D-NMR, 2D-NMR, and X-ray crystallographic analysis in case of compound 3. All isolated compounds were evaluated for their antimicrobial activity against a panel of clinically significant microorganisms. Compounds 1 and 3 selectively inhibited Enterococcus faecalis (MIC: 256 mug/mL). Compound 2 was found to have antibacterial and antifungal activity against Escherichia coli (MIC: 64 mug/mL), Salmonella enterica (MIC: 256 mug/mL), Staphylococcus aureus (MIC: 256 mug/mL), Enterococcus faecalis (MIC: 256 mug/mL), and Candida albicans (MIC: 64 mug/mL). Except for compounds 9 and 10, the other known metabolites (4-8) also exhibited antimicrobial activity.


