AcuminatinCAS# 41744-39-2 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 41744-39-2 | SDF | Download SDF |
| PubChem ID | 6441048 | Appearance | Cryst. |
| Formula | C21H24O4 | M.Wt | 340.4 |
| Type of Compound | Lignans | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
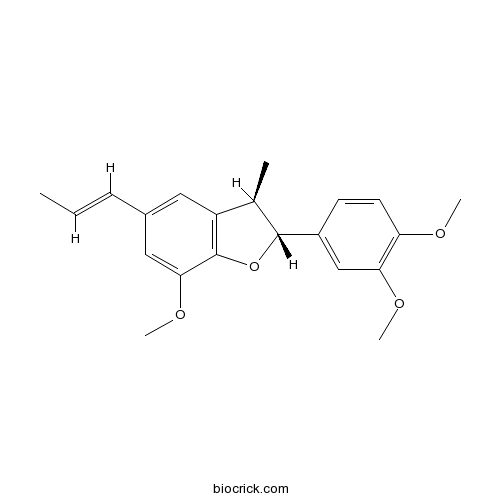
| Chemical Name | (2R,3R)-2-(3,4-dimethoxyphenyl)-7-methoxy-3-methyl-5-[(E)-prop-1-enyl]-2,3-dihydro-1-benzofuran | ||
| SMILES | CC=CC1=CC2=C(C(=C1)OC)OC(C2C)C3=CC(=C(C=C3)OC)OC | ||
| Standard InChIKey | ITFKWUHXYCXXFF-XSOBDOKWSA-N | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | 1. (+)-trans-Acuminatin, and (+)-cis-acuminatin show weak activity against platelet aggregation with IC50 values of 108.5 and 90.02 uM, respectively. 2. (-)-Acuminatin, and machilin G show dose-dependent potent inhibitory activities against PLCgamma1 in vitro with IC50 values ranging from 8.8 to 26.0 microM, the inhibition of PLCgamma1 may be an important mechanism for an antiproliferative effect on the human cancer cells, therefore, these inhibitors may be utilized as cancer chemotherapeutic and chemopreventive agents. 3. (-)-Acuminatin exerts hepatoprotective activities, perhaps by serving as a potent antioxidant. |
| Targets | PAFR |

Acuminatin Dilution Calculator

Acuminatin Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.9377 mL | 14.6886 mL | 29.3772 mL | 58.7544 mL | 73.443 mL |
| 5 mM | 0.5875 mL | 2.9377 mL | 5.8754 mL | 11.7509 mL | 14.6886 mL |
| 10 mM | 0.2938 mL | 1.4689 mL | 2.9377 mL | 5.8754 mL | 7.3443 mL |
| 50 mM | 0.0588 mL | 0.2938 mL | 0.5875 mL | 1.1751 mL | 1.4689 mL |
| 100 mM | 0.0294 mL | 0.1469 mL | 0.2938 mL | 0.5875 mL | 0.7344 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- (-)-Sativan
Catalog No.:BCN7752
CAS No.:41743-86-6
- Irisflorentin
Catalog No.:BCN1278
CAS No.:41743-73-1
- Pyrochamissanthin
Catalog No.:BCN3612
CAS No.:41743-60-6
- Sugeroside
Catalog No.:BCN6961
CAS No.:41743-57-1
- Luteone
Catalog No.:BCN5476
CAS No.:41743-56-0
- Procyanidin A2
Catalog No.:BCN6805
CAS No.:41743-41-3
- Bavachromene
Catalog No.:BCN3191
CAS No.:41743-38-8
- Croalbidine
Catalog No.:BCN2068
CAS No.:41714-30-1
- Indicine N-oxide
Catalog No.:BCN1996
CAS No.:41708-76-3
- Epimagnolin A
Catalog No.:BCN7831
CAS No.:41689-51-4
- Epiaschantin
Catalog No.:BCN7206
CAS No.:41689-50-3
- 8-Acetoxypentadeca-1,9Z-diene-4,6-diyn-3-ol
Catalog No.:BCN1444
CAS No.:41682-30-8
- Ginsenoside Rb1
Catalog No.:BCN1063
CAS No.:41753-43-9
- Ophiopogonin D
Catalog No.:BCN5004
CAS No.:41753-55-3
- Dihydrophaseic acid
Catalog No.:BCN5478
CAS No.:41756-77-8
- Evonimine
Catalog No.:BCN3082
CAS No.:41758-69-4
- ar-Curcumene
Catalog No.:BCN7534
CAS No.:4176-06-1
- 14-Deoxyandrographolide
Catalog No.:BCN3706
CAS No.:4176-97-0
- Lenvatinib (E7080)
Catalog No.:BCC1172
CAS No.:417716-92-8
- H-Leu-pNA.HCl
Catalog No.:BCC2972
CAS No.:4178-93-2
- Hypecorinine
Catalog No.:BCN3298
CAS No.:41787-57-9
- Junicedric acid
Catalog No.:BCN7660
CAS No.:41787-69-3
- Hesperetin-7-methyl ether
Catalog No.:BCN8501
CAS No.:
- Mangiferolic acid
Catalog No.:BCN4636
CAS No.:4184-34-3
Amides and neolignans from the aerial parts of Piper bonii.[Pubmed:27452451]
Phytochemistry. 2016 Sep;129:36-44.
Six amides, piperbonamides A-F, three neolignans piperbonins A-C, and 11 known compounds were isolated from the aerial parts of Piper bonii (Piperaceae). The structures of piperbonamides A-F and piperbonins A-C were elucidated based on the analysis of 1D and 2D NMR and MS data. Piperbonin A, (+)-trans-Acuminatin, (+)-cis-Acuminatin, (+)-kadsurenone, and pipernonaline showed weak activity against platelet aggregation with IC50 values of 118.2, 108.5, 90.02, 107.3, and 116.3 muM, respectively, as compared with the positive control, tirofiban, with an IC50 value of 5.24 muM. Piperbonamides A-F were inactive against five tumor cell lines at concentrations up to 40 muM.
Antioxidant lignans from Machilus thunbergii protect CCl4-injured primary cultures of rat hepatocytes.[Pubmed:11045899]
J Pharm Pharmacol. 2000 Sep;52(9):1163-9.
Eleven lignans (1-11) were isolated from the CH2Cl2 fraction of the bark of Machilus thunbergii Sieb. et Zucc. (Lauraceae). These were identified as (-)-Acuminatin (1), (-)-isoguaiacin (2), meso-dihydroguaiaretic acid (3), (+)-galbacin (4), (-)-sesamin (5), (+)-galbelgin (6), machilin A (7), machilin G (8), licarin A (9), and nectandrin A (10) and B (11). Primary cultures of rat hepatocytes were co-incubated for 90 min with the hepatotoxin CCl4 and each of the 11 lignans (50 microM). Hepatoprotective activity was determined by measuring the level of glutamic pyruvic transaminase released into the medium from the primary cultures of rat hepatocytes. (-)-Acuminatin, (-)-isoguaiacin and meso-dihydroguaiaretic acid all significantly reduced the level of glutamic pyruvic transaminase released. Further investigation revealed that these three compounds significantly preserved the levels and the activities of glutathione, superoxide dismutase, glutathione peroxidase and catalase. (-)-Acuminatin, (-)-isoguaiacin and meso-dihydroguaiaretic acid also ameliorated lipid peroxidation as demonstrated by a reduction of malondialdehyde production. These results suggest that (-)-Acuminatin, (-)-isoguaiacin and meso-dihydroguaiaretic acid exert diverse hepatoprotective activities, perhaps by serving as potent antioxidants.
Inhibition of phospholipase Cgamma1 and cancer cell proliferation by lignans and flavans from Machilus thunbergii.[Pubmed:15554262]
Arch Pharm Res. 2004 Oct;27(10):1043-7.
Thirteen compounds were isolated from the CH2Cl2 fraction of Machilus thunbergii as phospholipase Cgamma1 (PLCgamma1) inhibitors. These compounds were identified as nine lignans, two neolignans, and two flavans by spectroscopic analysis. Of these, 5,7-di-O-methyl-3',4'-methylenated (-)-epicatechin (12) and 5,7,3'-tri-O-methyl (-)-epicatechin (13) have not been reported previously in this plant. In addition, seven compounds, machilin A (1), (-)-sesamin (3), machilin G (5), (+)-galbacin (9), licarin A (10), (-)-Acuminatin (11) and compound 12 showed dose-dependent potent inhibitory activities against PLCgamma1 in vitro with IC50 values ranging from 8.8 to 26.0 microM. These lignans, neolignans, and flavans are presented as a new class of PLCgamma1 inhibitors. The brief study of the structure activity relationship of these compounds suggested that the benzene ring with the methylene dioxy group is responsible for the expression of inhibitory activities against PLCgamma1. Moreover, it is suggested that inhibition of PLCgamma1 may be an important mechanism for an antiproliferative effect on the human cancer cells. Therefore, these inhibitors may be utilized as cancer chemotherapeutic and chemopreventive agents.


