Amlodipine BesylateBlock of L-type calcium channel CAS# 111470-99-6 |
- Azelnidipine
Catalog No.:BCC4400
CAS No.:123524-52-7
- Verapamil HCl
Catalog No.:BCC4747
CAS No.:152-11-4
- Gabapentin HCl
Catalog No.:BCC4502
CAS No.:60142-95-2
- Zonisamide sodium
Catalog No.:BCC4240
CAS No.:68291-98-5
- Manidipine
Catalog No.:BCC4404
CAS No.:89226-50-6
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 111470-99-6 | SDF | Download SDF |
| PubChem ID | 60496 | Appearance | Powder |
| Formula | C26H31ClN2O8S | M.Wt | 567.05 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | Norvasc | ||
| Solubility | DMSO : ≥ 45 mg/mL (79.36 mM) H2O : 1 mg/mL (1.76 mM; Need ultrasonic) *"≥" means soluble, but saturation unknown. | ||
| Chemical Name | 2-[(2-Aminoethoxy)methyl]-4-(2-chlo | ||
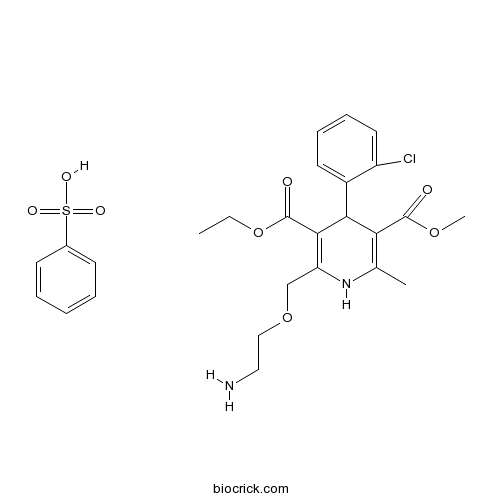
| SMILES | CCOC(=O)C1=C(COCCN)NC(=C(C1c2ccccc2Cl)C(=O)OC)C.O[S](=O)(=O)c3ccccc3 | ||
| Standard InChIKey | ZPBWCRDSRKPIDG-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C20H25ClN2O5.C6H6O3S/c1-4-28-20(25)18-15(11-27-10-9-22)23-12(2)16(19(24)26-3)17(18)13-7-5-6-8-14(13)21;7-10(8,9)6-4-2-1-3-5-6/h5-8,17,23H,4,9-11,22H2,1-3H3;1-5H,(H,7,8,9) | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | L-type calcium channel blocker that displays antihypertensive properties. Inhibits Ca2+-induced contractions in depolarized rat aorta (IC50 = 1.9 nM) and displays vasoprotective effects in cardiovascular disease. Inhibits proliferation of human vascular smooth muscle cells and epidermoid carcinoma A431 cells (IC50 = 25 μM). |

Amlodipine Besylate Dilution Calculator

Amlodipine Besylate Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 1.7635 mL | 8.8176 mL | 17.6351 mL | 35.2703 mL | 44.0878 mL |
| 5 mM | 0.3527 mL | 1.7635 mL | 3.527 mL | 7.0541 mL | 8.8176 mL |
| 10 mM | 0.1764 mL | 0.8818 mL | 1.7635 mL | 3.527 mL | 4.4088 mL |
| 50 mM | 0.0353 mL | 0.1764 mL | 0.3527 mL | 0.7054 mL | 0.8818 mL |
| 100 mM | 0.0176 mL | 0.0882 mL | 0.1764 mL | 0.3527 mL | 0.4409 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Amlodipine besylate is a long-acting Ca2+ channel blocker that reduce blood pressure as an antihypertensive drugs.
Calcium channel is an ion channel that selectively permeable to calcium ions. It serves vital functions in cellular signal transduction.
In cortical neurons, treatment of amlodipine besylate (<= 5 um) rescued the viability of H(2) O(2) injured cell and elevated the free radicals levels. Amlodipine besylate exhibits neuroptorective effects, such as reducing oxidative stress, promoting survival signals and blocking death signals. [1] It also showed proliferative effect on human epidermoid carcinoma A431 cells.[2]
In patient with exertional angina, amlodipine besylate reduced the total peripheral resistance and myocardial oxygen demand. In cat orally treated with amlodipine besylate daily, the average indirect systolic blood pressure were decreased without significant body weight/serum creatinine/potassium concentrations changes. [3]
References:
[1] Lee YJ, Park HH, Koh SH, Choi NY, Lee KY. Amlodipine besylate and amlodipine camsylate prevent cortical neuronal cell death induced by oxidative stress. J Neurochem. 2011 Dec;119(6):1262-70. doi: 10.1111/j.1471-4159.2011.07529.x.
[2] Yoshida J, Ishibashi T, Nishio M. Antiproliferative effect of Ca2+ channel blockers on human epidermoid carcinoma A431 cells. Eur J Pharmacol. 2003 Jul 4;472(1-2):23-31.
[3] Henik RA, Snyder PS, Volk LM. Treatment of systemic hypertension in cats with amlodipine besylate. J Am Anim Hosp Assoc. 1997 May-Jun;33(3):226-34.
- Naltrindole hydrochloride
Catalog No.:BCC6773
CAS No.:111469-81-9
- Axinysone B
Catalog No.:BCN7713
CAS No.:1114491-60-9
- Pinocembrin diacetate
Catalog No.:BCN5997
CAS No.:111441-88-4
- Azadirachtin
Catalog No.:BCC8123
CAS No.:11141-17-6
- Zileuton
Catalog No.:BCC2515
CAS No.:111406-87-2
- Lestaurtinib
Catalog No.:BCC2440
CAS No.:111358-88-4
- Sappanol
Catalog No.:BCN3735
CAS No.:111254-19-4
- Episappanol
Catalog No.:BCN7940
CAS No.:111254-18-3
- CGS 20625
Catalog No.:BCC7375
CAS No.:111205-55-1
- 7,3',4'-Trihydroxy-3-benzyl-2H-chromene
Catalog No.:BCN1621
CAS No.:1111897-60-9
- 1,2-O-Dilinoleoyl-3-O-beta-D-galactopyranosylracglycerol
Catalog No.:BCN6768
CAS No.:111187-15-6
- PF-04880594
Catalog No.:BCC3998
CAS No.:1111636-35-1
- 25-Anhydroalisol F
Catalog No.:BCN3361
CAS No.:1114895-01-0
- Ac-DL-Met-OH
Catalog No.:BCC2999
CAS No.:1115-47-5
- H-Ala-OEt.HCl
Catalog No.:BCC2687
CAS No.:1115-59-9
- L-Cysteinesulfinic acid
Catalog No.:BCC6571
CAS No.:1115-65-7
- Metformin HCl
Catalog No.:BCC4799
CAS No.:1115-70-4
- Nyasicoside
Catalog No.:BCN5998
CAS No.:111518-94-6
- Nyasicol
Catalog No.:BCN5999
CAS No.:111518-95-7
- Fmoc-D-Phg-OH
Catalog No.:BCC3316
CAS No.:111524-95-9
- 3',5-Dihydroxy-4',5',6,7-tetramethoxyflavone
Catalog No.:BCN1620
CAS No.:111537-41-8
- Anonamine
Catalog No.:BCN2139
CAS No.:111566-66-6
- Cyanidin-3-O-arabinoside chloride
Catalog No.:BCN3023
CAS No.:111613-04-8
- GSK1838705A
Catalog No.:BCC3787
CAS No.:1116235-97-2
Comparisons of the pharmacokinetics and tolerability of fixed-dose combinations of amlodipine besylate/losartan and amlodipine camsylate/losartan in healthy subjects: a randomized, open-label, single-dose, two-period, two-sequence crossover study.[Pubmed:27703330]
Drug Des Devel Ther. 2016 Sep 20;10:3021-3028.
BACKGROUND: A fixed-dose combination (FDC) of amlodipine and losartan has been used to reduce blood pressure in patients whose hypertension is not sufficiently controlled with either drug alone. The aim of this study was to evaluate the pharmacokinetic (PK) characteristics and tolerability of an FDC of 6.94 mg Amlodipine Besylate (5 mg as amlodipine)/50 mg losartan potassium compared to an FDC of 5 mg amlodipine camsylate/50 mg losartan potassium in healthy subjects. SUBJECTS AND METHODS: A randomized, open-label, single-dose, two-period, two-sequence crossover study was conducted on 46 healthy male subjects. Blood concentrations were measured by liquid chromatography-tandem mass spectrometry. Blood samples were collected up to 144 hours post dose for each period. PK parameters were calculated in each treatment group using a noncompartmental method. The 90% confidence intervals (CIs) of the geometric mean ratios of the two treatments for the maximum plasma concentration (Cmax) and the area under the concentration curve from time zero to the last quantifiable time point (AUC0-t) were estimated. Tolerability assessments were performed for all subjects who received the drug at least once. RESULTS: The PK profiles of the two treatments were similar. For amlodipine, the geometric mean ratios (90% CIs) of Amlodipine Besylate to amlodipine camsylate for the Cmax and AUC0-t were 0.98 (0.94-1.01) and 0.97 (0.93-1.01), respectively. The corresponding values for losartan were 0.91 (0.81-1.02) and 1.05 (0.98-1.12), respectively. The incidence of adverse events was not significantly different between the two treatments, and both were well tolerated. CONCLUSION: An FDC of 6.94 mg Amlodipine Besylate (5 mg as amlodipine)/50 mg losartan potassium produced similar results to an FDC of 5 mg amlodipine camsylate/50 mg losartan potassium treatment with respect to the PK parameters of amlodipine and losartan based on Cmax and AUC0-t values. The Amlodipine Besylate/losartan potassium combination was well tolerated by healthy male subjects.
Molecular insight into atypical instability behavior of fixed-dose combination containing amlodipine besylate and losartan potassium.[Pubmed:28064090]
J Pharm Biomed Anal. 2017 Mar 20;136:66-80.
Combination therapy with the use of fixed-dose combinations (FDCs) is evincing increasing interest of prescribers, manufacturers and even regulators, evidently due to the primary benefit of improved patient compliance. However, owing to potential of drug-drug interaction, FDCs require closer scrutiny with respect to their physical and chemical stability. Accordingly, the purpose of the present study was to explore stability behavior of a popular antihypertensive combination of Amlodipine Besylate (AML) and losartan potassium (LST). Physical mixtures of the two drugs and multiple marketed formulations were stored under accelerated conditions of temperature and humidity (40 degrees C/75% RH) in a stability chamber and samples were withdrawn after 1 and 3 months. The physical changes were observed visibly, while chemical changes were monitored by HPLC employing a method that could separate the two drugs and all other components present. The combination revealed strong physical instability and also chemical degradation of AML in the presence of LST. Interestingly, three isomeric interaction products of AML were formed in the combination, which otherwise were reported in the literature to be generated on exposure of AML free base above its melting point. The same unusual products were even formed when multiple marketed FDCs were stored under accelerated conditions outside their storage packs. However, these were absent when AML alone was stored in the same studied conditions. Therefore, reasons for physical and chemical incompatibility and the mechanism of degradation of AML in the presence of LST were duly explored at the molecular level. The outcomes of the study are expected to help in development of stable FDCs of the two drugs.
Conductive Polymeric Ionic Liquid/Fe3O4 Nanocomposite as an Efficient Catalyst for the Voltammetric Determination of Amlodipine Besylate.[Pubmed:28118570]
J AOAC Int. 2017 Mar 1;100(2):406-413.
A novel conductive polymeric ionic liquid (IL)-Fe3O4 nanocomposite (represented as PIL-Fe3O4) based on inorganic-organic hybrid material was synthesized using two different methods. Nuclear magnetic resonance, Fourier transform infrared, X-ray diffraction, and field emission scanning electron microscopy characterized the structures of IL, Fe3O4 nanoparticles, and PIL-Fe3O4. The electrochemical sensors based on PIL-Fe3O4-modified glassy carbon electrode were fabricated, and each of these nanocomposites was examined for the ability to determine Amlodipine Besylate (AMD). The electrochemical study of the modified electrodes, as well as its efficiency for the electro-oxidation of AMD, was described in 0.1 M phosphate-buffered solution (pH 7.0) using voltammetric methods. The results exhibit a linear dynamic range from 1 to 500 nM and a detection limit of 0.36 nM. Finally, the modified electrode was used for the determination of AMD in pharmaceutical and biological samples.
Calcium channel blockades exhibit anti-inflammatory and antioxidative effects by augmentation of endothelial nitric oxide synthase and the inhibition of angiotensin converting enzyme in the N(G)-nitro-L-arginine methyl ester-induced hypertensive rat aorta: vasoprotective effects beyond the blood pressure-lowering effects of amlodipine and manidipine.[Pubmed:16392774]
Hypertens Res. 2005 Aug;28(8):689-700.
Long-acting dihydropyridine calcium channel blockades have been shown to limit the progression of atherosclerosis and decrease the incidence of cardiovascular events in humans and animals. To investigate the vasoprotective effects beyond the blood pressure-lowering effects of these agents, amlodipine (20 mg/kg/ day) and manidipine (10 mg/kg/day) were administered by gavage to N(G)-nitro-L-arginine methyl ester (L-NAME)-induced hypertensive rats for 2 weeks. L-NAME treatment (0.7 mg/ml in drinking water) significantly decreased the gene and protein expression of endothelial nitric oxide synthase (eNOS) and increased nicotinamide adenine dinucleotide phosphate (NADPH) oxidase, vascular cell adhesion molecule-1 (VCAM-1), and monocyte chemoattractant protein-1 (MCP-1) mRNA levels in the aorta, as determined by Western blotting and reverse transcription (RT)-polymerase chain reaction (PCR). Amlodipine and manidipine normalized the decreased expression of eNOS gene and protein, and attenuated the overexpression of NADPH oxidase, VCAM-1, and MCP-1 mRNA. Furthermore, amlodipine and manidipine prevented the L-NAME-induced increase in the angiotensin converting enzyme (ACE) mRNA content, thereby restoring control levels in the aorta. On the other hand, hydralazine treatment had no such effect in L-NAME treated rats. Furthermore, the increased expression of manganese superoxide dismutase (Mn-SOD) by L-NAME treatment was not affected by amlodipine, manidipine, or hydralazine. We concluded that the direct anti-inflammatory and antioxidative effects of calcium channel blockades in the aorta of rats with L-NAME-induced hypertension were not likely to have been mediated by the blood pressure-lowering action of these agents, but instead these beneficial effects appear to have been mediated by an augmentation of eNOS expression and by the inhibition of the expression of ACE.
Antiproliferative effect of Ca2+ channel blockers on human epidermoid carcinoma A431 cells.[Pubmed:12860469]
Eur J Pharmacol. 2003 Jul 4;472(1-2):23-31.
The effects of Ca(2+) channel blockers on the proliferation of human epidermoid carcinoma A431 cells were investigated by microtiter tetrazolium (MTT) proliferation assay and bromodeoxyuridine (BrdU) incorporation assay. Dihydropyridine derivatives, such as amlodipine, nicardipine, and nimodipine inhibited A431 cell growth and the incorporation of BrdU into cells with IC(50) values of 20-30 microM, while verapamil, diltiazem and dihydropyridine nifedipine inhibited neither the cell growth nor BrdU incorporation at the same concentration. Though extracellular Ca(2+) is indispensable to the cell growth, an L-type Ca(2+) channel agonist, 1,4-dihydro-2,6-dimethyl-5-nitro-4-[2-(trifluoromethyl) phenyl]pyridine-3-carboxylic acid methyl ester (200 nM), did not affect the antiproliferative action of amlodipine. Thapsigargin, an inhibitor of Ca(2+)-ATPase of the endoplasmic reticulum, inhibited itself the growth of A431 cells and also showed a synergistic effect with the antiproliferative action of amlodipine. In the fluorimetric measurement of intracellular free Ca(2+) concentration in fura-2 or fluo-3 loaded A431 cells, amlodipine blunted the thapsigargin- or cyclopiazonic acid-induced Ca(2+) release from endoplasmic reticulum and the ensuing Ca(2+) influx through Ca(2+)-permeable channels. The effect on the thapsigargin-induced Ca(2+) responses could be reproduced by nicardipine and nimodipine but not by nifedipine or verapamil, lacking antiproliferative potency. These findings suggest that the intracellular Ca(2+) control system responsible for thapsigargin- and cyclopiazonic acid-sensitive endoplasmic reticulum, but not L-type Ca(2+) channels, may be modulated by amlodipine, which results in the inhibition of A431 cell growth.
Calcium channel blocking properties of amlodipine in vascular smooth muscle and cardiac muscle in vitro: evidence for voltage modulation of vascular dihydropyridine receptors.[Pubmed:2434785]
J Cardiovasc Pharmacol. 1987 Jan;9(1):110-9.
Amlodipine was twice as potent as nifedipine at inhibiting Ca2+-induced contractions in depolarised rat aorta (IC50 1.9 nM vs. 4.1 nM) but, unlike nifedipine, displayed a very slow onset of action. Contractions induced by depolarising steps with 45 mM K+ were much less potently blocked by amlodipine (IC50 19.4 nM), whereas the potency of nifedipine was little changed (IC50 7.1 nM). This difference may be explained by a modulated receptor hypothesis, similar to that described for cardiac muscle, in which block of vascular calcium channels by dihydropyridines is enhanced at depolarized membrane potentials, such voltage-dependence only being apparent with a slow-acting drug such as amlodipine. Recovery from amlodipine block of K+-responses in rat portal vein after drug washout was also very slow. Amlodipine and nifedipine blocked phenylephrine-induced contractions of the rat aorta with potencies similar to those against depolarisation-induced responses. Negative inotropic potencies of amlodipine and nifedipine in perfused guinea pig hearts were approximately one-tenth those against Ca2+-induced contractions in rat aorta. Amlodipine caused complete block of guinea pig papillary muscle single-cell slow action potentials at a concentration (5 microM) that had no effect on upstroke velocity of normal, fast potentials but reduced the duration of the plateau phase.


