AnisomycinJNK agonist, potent and specific CAS# 22862-76-6 |
- Cefditoren Pivoxil
Catalog No.:BCC4898
CAS No.:117467-28-4
- Cefoselis
Catalog No.:BCC4092
CAS No.:122841-10-5
- Balofloxacin
Catalog No.:BCC4892
CAS No.:127294-70-6
- Pefloxacin Mesylate Dihydrate
Catalog No.:BCC5089
CAS No.:149676-40-4
- Tinidazole
Catalog No.:BCC4866
CAS No.:19387-91-8
- Toltrazuril
Catalog No.:BCC4870
CAS No.:69004-03-1
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 22862-76-6 | SDF | Download SDF |
| PubChem ID | 31549 | Appearance | Powder |
| Formula | C14H19NO4 | M.Wt | 265.31 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | Flagecidin; Wuningmeisu C | ||
| Solubility | DMSO : ≥ 50 mg/mL (188.46 mM) *"≥" means soluble, but saturation unknown. | ||
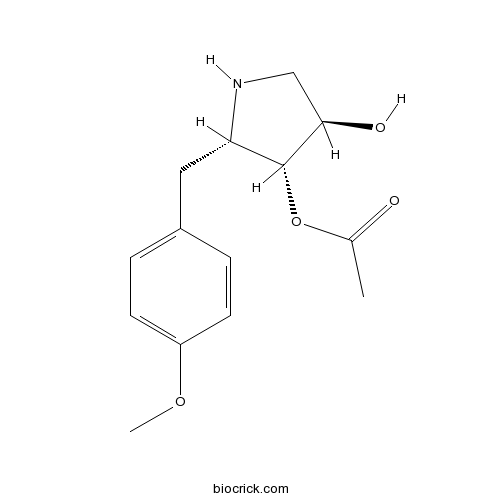
| Chemical Name | [(2S,3R,4R)-4-hydroxy-2-[(4-methoxyphenyl)methyl]pyrrolidin-3-yl] acetate | ||
| SMILES | CC(=O)OC1C(CNC1CC2=CC=C(C=C2)OC)O | ||
| Standard InChIKey | YKJYKKNCCRKFSL-BFHYXJOUSA-N | ||
| Standard InChI | InChI=1S/C14H19NO4/c1-9(16)19-14-12(15-8-13(14)17)7-10-3-5-11(18-2)6-4-10/h3-6,12-15,17H,7-8H2,1-2H3/t12-,13+,14+/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Protein synthesis inhibitor (blocks translation). Potent activator of stress-activated protein kinases (JNK/SAPK) and p38 MAP kinase. Acts as a potent signaling agonist to selectively elicit homologous desensitization of immediate early gene induction (c-fos, fosB, c-jun, junB and junD). |

Anisomycin Dilution Calculator

Anisomycin Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.7692 mL | 18.8459 mL | 37.6918 mL | 75.3835 mL | 94.2294 mL |
| 5 mM | 0.7538 mL | 3.7692 mL | 7.5384 mL | 15.0767 mL | 18.8459 mL |
| 10 mM | 0.3769 mL | 1.8846 mL | 3.7692 mL | 7.5384 mL | 9.4229 mL |
| 50 mM | 0.0754 mL | 0.3769 mL | 0.7538 mL | 1.5077 mL | 1.8846 mL |
| 100 mM | 0.0377 mL | 0.1885 mL | 0.3769 mL | 0.7538 mL | 0.9423 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Anisomycin is a specific agonist of JNK with a concentration of 25 ng/ml [1].
JNK is short for c-Jun N-terminal kinase which reported as a proapoptotic kinase and plays an important role in many cellular events, such as cell cycle, proliferation, apoptosis and cell stress. It is also reported that JNK plays a pivotal role in the cell apoptosis induced by UV and activated JNK pathway could enhance TNF-α mediated apoptosis thus often regarded as a potent target in clinic [2] [3].
Anisomycin is a potent JNK agonist. When tested with hormone refractory cell line DU 145(highly resist to Fas mediated apoptosis), 250 ng/ml anisomysin treatment induced DU145 cells apoptosis together with Fas (200 ng/ml) via activating JNK [4]. In HL-60 cells, treatment of anisomysin activated JNK pathway activity which further induced cell apoptosis [5]. When tested with primary murine embryonic fibroblasts, anisomycin treatment stimulated cell apoptosis via activating JNK expression [6].
References:
[1]. Jiang, J., et al., Spermassociated antigen 9 promotes astrocytoma cell invasion through the upregulation of podocalyxin. Mol Med Rep, 2014. 10(1): p. 417-22.
[2]. Lin, A., Activation of the JNK signaling pathway: breaking the brake on apoptosis. Bioessays, 2003. 25(1): p. 17-24.
[3]. Liu, J. and A. Lin, Role of JNK activation in apoptosis: a double-edged sword. Cell Res, 2005. 15(1): p. 36-42.
[4]. Curtin, J.F. and T.G. Cotter, Anisomycin activates JNK and sensitises DU 145 prostate carcinoma cells to Fas mediated apoptosis. Br J Cancer, 2002. 87(10): p. 1188-94.
[5]. Stadheim, T.A. and G.L. Kucera, c-Jun N-terminal kinase/stress-activated protein kinase (JNK/SAPK) is required for mitoxantrone- and anisomycin-induced apoptosis in HL-60 cells. Leuk Res, 2002. 26(1): p. 55-65.
[6]. Tournier, C., et al., Requirement of JNK for stress-induced activation of the cytochrome c-mediated death pathway. Science, 2000. 288(5467): p. 870-4.
- Ki8751
Catalog No.:BCC1116
CAS No.:228559-41-9
- VULM 1457
Catalog No.:BCC7533
CAS No.:228544-65-8
- 9-Epiblumenol B
Catalog No.:BCN5075
CAS No.:22841-42-5
- Pratensein
Catalog No.:BCN2918
CAS No.:2284-31-3
- Aspartame
Catalog No.:BCC8836
CAS No.:22839-47-0
- Boc-D-Val-OH
Catalog No.:BCC3466
CAS No.:22838-58-0
- Miconazole nitrate
Catalog No.:BCC9047
CAS No.:22832-87-7
- Hypoglaunine A
Catalog No.:BCN3086
CAS No.:228259-16-3
- Boc-Met-OH.DCHA
Catalog No.:BCC2602
CAS No.:22823-50-3
- Alisol K 23-acetate
Catalog No.:BCN3363
CAS No.:228095-18-9
- 3-(4-Hydroxy-3,5-dimethoxyphenyl)-1,2-propanediol
Catalog No.:BCN1480
CAS No.:22805-15-8
- 1,2,3,7-Tetramethoxyxanthone
Catalog No.:BCN7519
CAS No.:22804-52-0
- 6-Acetonyldihydrochelerythrine
Catalog No.:BCN5076
CAS No.:22864-92-2
- Famprofazone
Catalog No.:BCC3779
CAS No.:22881-35-2
- Silymarin
Catalog No.:BCN6299
CAS No.:22888-70-6
- 4-Amino-3,5-dichloropyridine
Catalog No.:BCC8679
CAS No.:22889-78-7
- TAK-779
Catalog No.:BCC4137
CAS No.:229005-80-5
- R 892
Catalog No.:BCC5992
CAS No.:229030-05-1
- Ginkgolic acid C15:1
Catalog No.:BCN2307
CAS No.:22910-60-7
- Neferine
Catalog No.:BCN6338
CAS No.:2292-16-2
- Desmethoxycentaureidin
Catalog No.:BCN5077
CAS No.:22934-99-2
- Abn-CBD
Catalog No.:BCC7011
CAS No.:22972-55-0
- GW9662
Catalog No.:BCC2260
CAS No.:22978-25-2
- AGN 194310
Catalog No.:BCC5416
CAS No.:229961-45-9
The developmental competence of oocytes parthenogenetically activated by an electric pulse and anisomycin treatment.[Pubmed:27864653]
Biotechnol Lett. 2017 Feb;39(2):189-196.
OBJECTIVE: The aim of this study was to investigate the developmental competence of oocytes parthenogenetically activated by an electric pulse (EP) and treated with Anisomycin and to determine whether this method is applicable to somatic cell nuclear transfer (SCNT). RESULTS: Embryos derived from porcine oocytes parthenogenetically activated by an EP and treatment with 0.01 microg/mL Anisomycin had a significantly improved in vitro developmental capacity. Furthermore, 66.6% of blastocysts derived from these embryos had a diploid karyotype. The blastocyst formation rate of cloned embryos was similar between oocytes activated by an EP and treated with 2 mM 6-dimethylaminopurine for 4 h and those activated by an EP and treated with 0.01 microg/mL Anisomycin for 4 h. The level of maturation-promoting factor was significantly decreased in oocytes activated by an EP and treated with Anisomycin. Finally, the mRNA expression levels of apoptosis-related genes (Bax and Bcl-2) and pluripotency-related genes (Oct4, Nanog, and Sox2) were checked by RT-PCR. CONCLUSION: Our results demonstrate that porcine oocyte activation via an EP in combination with Anisomycin treatment can lead to a high blastocyst formation rate in parthenogenetic activation and SCNT experiments.
Anisomycin-induced GATA-6 degradation accompanying a decrease of proliferation of colorectal cancer cell.[Pubmed:27404124]
Biochem Biophys Res Commun. 2016 Sep 9;478(1):481-485.
Transcription factor GATA-6 plays a key role in normal cell differentiation of the mesoderm and endoderm. On the other hand, GATA-6 is abnormally overexpressed in many clinical gastrointestinal cancer tissue samples, and accelerates cell proliferation or an anti-apoptotic response in cancerous tissues. We previously showed that activation of the JNK signaling cascade causes proteolysis of GATA-6. In this study, we demonstrated that Anisomycin, a JNK activator, stimulates nuclear export of GATA-6 in a colorectal cancer cell line, DLD-1. Concomitantly, Anisomycin remarkably inhibits the proliferation of DLD-1 cells via G2/M arrest in a plate culture. However, it did not induce apoptosis under growth arrest conditions. Furthermore, the growth of DLD-1 cells in a spheroid culture was suppressed by Anisomycin. Although 5-FU showed only a slight inhibitory effect on 3D spheroid cultures, the same concentration of 5-FU together with a low concentration of Anisomycin exhibited strong growth inhibition. These results suggest that the induction of GATA-6 dysfunction may be more effective for chemotherapy for colorectal cancer, although the mechanism underlying the synergistic effect of 5-FU and Anisomycin remains unknown.
Biosynthesis of the pyrrolidine protein synthesis inhibitor anisomycin involves novel gene ensemble and cryptic biosynthetic steps.[Pubmed:28373542]
Proc Natl Acad Sci U S A. 2017 Apr 18;114(16):4135-4140.
The protein synthesis inhibitor Anisomycin features a unique benzylpyrrolidine system and exhibits diverse biological and pharmacologic activities. Its biosynthetic origin has remained obscure for more than 60 y, however. Here we report the identification of the biosynthetic gene cluster (BGC) of Anisomycin in Streptomyces hygrospinosus var. beijingensis by a bioactivity-guided high-throughput screening method. Using a combination of bioinformatic analysis, reverse genetics, chemical analysis, and in vitro biochemical assays, we have identified a core four-gene ensemble responsible for the synthesis of the pyrrolidine system in Anisomycin: aniQ, encoding a aminotransferase that catalyzes an initial deamination and a later reamination steps; aniP, encoding a transketolase implicated to bring together an glycolysis intermediate with 4-hydroxyphenylpyruvic acid to form the Anisomycin molecular backbone; aniO, encoding a glycosyltransferase that catalyzes a cryptic glycosylation crucial for downstream enzyme processing; and aniN, encoding a bifunctional dehydrogenase that mediates multistep pyrrolidine formation. The results reveal a BGC for pyrrolidine alkaloid biosynthesis that is distinct from known bacterial alkaloid pathways, and provide the signature sequences that will facilitate the discovery of BGCs encoding novel pyrrolidine alkaloids in bacterial genomes. The biosynthetic insights from this study further set the foundation for biosynthetic engineering of pyrrolidine antibiotics.
Enhancement of death receptor 4-mediated apoptosis and cytotoxicity in renal cell carcinoma cells by anisomycin.[Pubmed:27879498]
Anticancer Drugs. 2017 Feb;28(2):180-186.
Renal cell carcinoma (RCC) is one of the most drug-resistant malignancies, and an effective therapy is lacking for metastatic RCC. Anisomycin is known to inhibit protein synthesis and induce ribotoxic stress. The aim of this study was to explore whether Anisomycin enhances the cytotoxic effects of mapatumumab, a human agonistic monoclonal antibody specific for death receptor 4 (DR4), in human RCC cells. We examined the cytotoxicity of Anisomycin alone and in combination with mapatumumab in human RCC cell lines and primary RCC cell cultures. RCC cells treated with Anisomycin showed cytotoxicity in a dose-dependent manner. Anisomyin in combination with mapatumumab showed a synergistic effect not only in two human RCC cell lines but also in five primary RCC cell cultures. The synergy between Anisomycin and mapatumumab for cytotoxicity was also observed for apoptosis. Interestingly, Anisomycin significantly increased DR4 expression at both the mRNA and the protein level. Furthermore, the combination-induced cytotoxicity was significantly suppressed by a human recombinant DR4:Fc chimeric protein. The combination of Anisomycin and mapatumumab also enhanced the activity of caspases 8 and 3, the downstream molecules of death receptors. These findings indicate that Anisomycin sensitizes RCC cells to DR4-mediated apoptosis through the induction of DR4, suggesting that combinational treatment with Anisomycin and mapatumumab might represent a novel therapeutic strategy for the treatment of RCC.
The protein synthesis inhibitor anisomycin induces macrophage apoptosis in rabbit atherosclerotic plaques through p38 mitogen-activated protein kinase.[Pubmed:19286921]
J Pharmacol Exp Ther. 2009 Jun;329(3):856-64.
Because macrophages play a major role in atherosclerotic plaque destabilization, selective removal of macrophages represents a promising approach to stabilize plaques. We showed recently that the protein synthesis inhibitor cycloheximide, in contrast to puromycin, selectively depleted macrophages in rabbit atherosclerotic plaques without affecting smooth muscle cells (SMCs). The mechanism of action of these two translation inhibitors is dissimilar and could account for the differential effects on SMC viability. It is not known whether selective depletion of macrophages is confined to cycloheximide or whether it can also be achieved with translation inhibitors that have a similar mechanism of action. Therefore, in the present study, we investigated the effect of Anisomycin, a translation inhibitor with a mechanism of action similar to cycloheximide, on macrophage and SMC viability. In vitro, Anisomycin induced apoptosis of macrophages in a concentration-dependent manner, whereas SMCs were only affected at higher concentrations. In vivo, Anisomycin selectively decreased the macrophage content of rabbit atherosclerotic plaques through apoptosis. The p38 mitogen-activated protein kinase (MAPK) inhibitor SB202190 [4-(4-fluorophenyl)-2-(4-hydroxyphenyl)-5-(4-pyridyl)-1H-imidazole] prevented Anisomycin-induced macrophage death, without affecting SMC viability. SB202190 decreased Anisomycin-induced p38 MAPK phosphorylation, did not alter c-Jun NH(2)-terminal kinase (JNK) phosphorylation, and increased extracellular signal-regulated kinase (ERK) 1/2 phosphorylation. The latter effect was abolished by the mitogen-activated protein kinase kinase 1/2 inhibitor U0126 [1,4-diamino-2,3-dicyano-1,4-bis(2-aminophynyltio)butadiene ethanolate], although the prevention of Anisomycin-induced macrophage death by SB202190 remained unchanged. The JNK phosphorylation inhibitor SP600125 did not affect Anisomycin-induced macrophage or SMC death. In conclusion, Anisomycin selectively decreased the macrophage content in rabbit atherosclerotic plaques, indicating that this effect is not confined to cycloheximide. p38 MAPK, but not ERK1/2 or JNK, plays a major role in Anisomycin-induced macrophage death.
Anisomycin selectively desensitizes signalling components involved in stress kinase activation and fos and jun induction.[Pubmed:9528756]
Mol Cell Biol. 1998 Apr;18(4):1844-54.
Anisomycin, a translational inhibitor secreted by Streptomyces spp., strongly activates the stress-activated mitogen-activated protein (MAP) kinases JNK/SAPK (c-Jun NH2-terminal kinase/stress-activated protein kinase) and p38/RK in mammalian cells, resulting in rapid induction of immediate-early (IE) genes in the nucleus. Here, we have characterized this response further with respect to homologous and heterologous desensitization of IE gene induction and stress kinase activation. We show that Anisomycin acts exactly like a signalling agonist in eliciting highly specific and virtually complete homologous desensitization. Anisomycin desensitization of a panel of IE genes (c-fos, fosB, c-jun, junB, and junD), using epidermal growth factor (EGF), basic fibroblast growth factor, (bFGF), tumor necrosis factor alpha (TNF-alpha), Anisomycin, tetradecanoyl phorbol acetate (TPA), and UV radiation as secondary stimuli, was found to be extremely specific both with respect to the secondary stimuli and at the level of individual genes. Further, we show that Anisomycin-induced homologous desensitization is caused by the fact that Anisomycin no longer activates the JNK/SAPK and p38/RK MAP kinase cascades in desensitized cells. In Anisomycin-desensitized cells, activation of JNK/SAPKs by UV radiation and hyperosmolarity is almost completely lost, and that of the p38/RK cascade is reduced to about 50% of the normal response. However, all other stimuli produced normal or augmented activation of these two kinase cascades in Anisomycin-desensitized cells. These data show that Anisomycin behaves like a true signalling agonist and suggest that the Anisomycin-desensitized signalling component(s) is not involved in JNK/SAPK or p38/RK activation by EGF, bFGF, TNF-alpha, or TPA but may play a significant role in UV- and hyperosmolarity-stimulated responses.
Anisomycin-activated protein kinases p45 and p55 but not mitogen-activated protein kinases ERK-1 and -2 are implicated in the induction of c-fos and c-jun.[Pubmed:7935449]
Mol Cell Biol. 1994 Nov;14(11):7352-62.
Independent of its ability to block translation, Anisomycin intrinsically initiates intracellular signals and immediate-early gene induction [L. C. Mahadevan and D. R. Edwards, Nature (London) 349:747-749, 1991]. Here, we characterize further its action as a potent, selective signalling agonist. In-gel kinase assays show that epidermal growth factor (EGF) transiently activates five kinases: the mitogen-activated protein (MAP) kinases ERK-1 and -2, and three others, p45, p55, and p80. Anisomycin, at inhibitory and subinhibitory concentrations, does not activate ERK-1 and -2 but elicits strong sustained activation of p45 and p55, which are unique in being serine kinases whose detection is enhanced with poly-Glu/Tyr or poly-Glu/Phe copolymerized in these gels. Translational arrest using emetine or puromycin does not activate p45 and p55 but does prolong EGF-stimulated ERK-1 and -2 activation. Rapamycin, which blocks Anisomycin-stimulated p70/85S6k activation without affecting nuclear responses, has no effect on p45 or p55 kinase. p45 and p55 are activable by okadaic acid or UV irradiation, and both kinases phosphorylate the c-Jun NH2-terminal peptide 1-79, putatively placing them within c-Jun NH2-terminal kinase/stress-activated protein kinase (JNK/SAPK) subfamily of MAP kinases. Thus, the EGF- and Anisomycin-activated kinases p45 and p55 are strongly implicated in signalling to c-fos and c-jun, whereas the MAP kinases ERK-1 and -2 are not essential for this process.
The stress-activated protein kinase subfamily of c-Jun kinases.[Pubmed:8177321]
Nature. 1994 May 12;369(6476):156-60.
The mitogen-activated protein (MAP) kinases Erk-1 and Erk-2 are proline-directed kinases that are themselves activated through concomitant phosphorylation of tyrosine and threonine residues. The kinase p54 (M(r) 54,000), which was first isolated from cycloheximide-treated rats, is proline-directed like Erks-1/2, and requires both Tyr and Ser/Thr phosphorylation for activity. p54 is, however, distinct from Erks-1/2 in its substrate specificity, being unable to phosphorylate pp90rsk but more active in phosphorylating the c-Jun transactivation domain. Molecular cloning of p54 reveals a unique subfamily of extracellularly regulated kinases. Although they are 40-45% identical in sequence to Erks-1/2, unlike Erks-1/2 the p54s are only poorly activated in most cells by mitogens or phorbol esters. However, p54s are the principal c-Jun N-terminal kinases activated by cellular stress and tumour necrosis factor (TNF)-alpha, hence they are designated stress-activated protein kinases, or SAPKs. SAPKs are also activated by sphingomyelinase, which elicits a subset of cellular responses to TNF-alpha (ref. 9). SAPKs therefore define a new TNF-alpha and stress-activated signalling pathway, possibly initiated by sphingomyelin-based second messengers, which regulates the activity of c-Jun.
Role of SAPK/ERK kinase-1 in the stress-activated pathway regulating transcription factor c-Jun.[Pubmed:7997269]
Nature. 1994 Dec 22-29;372(6508):794-8.
The stress-activated protein kinases (SAPKs), which are distantly related to the MAP kinases, are the dominant c-Jun amino-terminal protein kinases activated in response to a variety of cellular stresses, including treatment with tumour-necrosis factor-alpha and interleukin-beta (refs 1, 2). SAPK phosphorylation of c-Jun probably activates the c-Jun transactivation function. SAPKs are part of a signal transduction cascade related to, but distinct from, the MAPK pathway. We have now identified a novel protein kinase, called SAPK/ERK kinase-1 (SEK1), which is structurally related to the MAP kinase kinases (MEKs). SEK1 is a potent activator of the SAPKs in vitro and in vivo. An inactive SEK1 mutant blocks SAPK activation by extracellular stimuli without interfering with the MAPK pathway. Although alternative mechanisms of SAPK activation may exist, as an immediate upstream activator of the SAPKs, SEK1 further defines a signalling cascade that couples cellular stress agonists to the c-Jun transcription factor.


