FamprofazoneCAS# 22881-35-2 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 22881-35-2 | SDF | Download SDF |
| PubChem ID | 3326 | Appearance | Powder |
| Formula | C24H31N3O | M.Wt | 377.52 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble in DMSO | ||
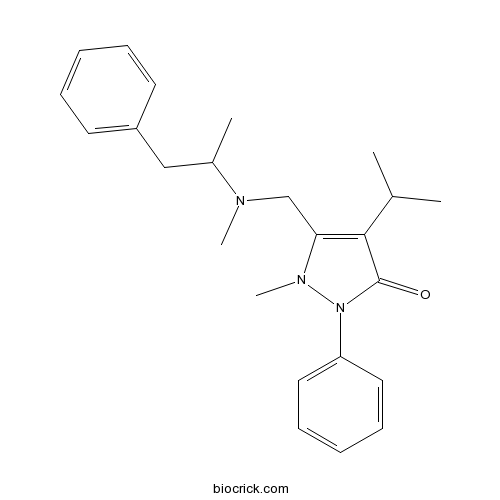
| Chemical Name | 1-methyl-5-[[methyl(1-phenylpropan-2-yl)amino]methyl]-2-phenyl-4-propan-2-ylpyrazol-3-one | ||
| SMILES | CC(C)C1=C(N(N(C1=O)C2=CC=CC=C2)C)CN(C)C(C)CC3=CC=CC=C3 | ||
| Standard InChIKey | GNUXVOXXWGNPIV-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C24H31N3O/c1-18(2)23-22(17-25(4)19(3)16-20-12-8-6-9-13-20)26(5)27(24(23)28)21-14-10-7-11-15-21/h6-15,18-19H,16-17H2,1-5H3 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Famprofazone is a non-steroidal anti-inflammatory agent (NSAID) of the pyrazolone series, has analgesic, anti-inflammatory, and antipyretic effects. |

Famprofazone Dilution Calculator

Famprofazone Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.6489 mL | 13.2443 mL | 26.4887 mL | 52.9773 mL | 66.2217 mL |
| 5 mM | 0.5298 mL | 2.6489 mL | 5.2977 mL | 10.5955 mL | 13.2443 mL |
| 10 mM | 0.2649 mL | 1.3244 mL | 2.6489 mL | 5.2977 mL | 6.6222 mL |
| 50 mM | 0.053 mL | 0.2649 mL | 0.5298 mL | 1.0595 mL | 1.3244 mL |
| 100 mM | 0.0265 mL | 0.1324 mL | 0.2649 mL | 0.5298 mL | 0.6622 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Famprofazone (Gewodin, Gewolen) is a non-steroidal anti-inflammatory agent (NSAID) of the pyrazolone series . It has analgesic, anti-inflammatory, and antipyretic effects.
- 6-Acetonyldihydrochelerythrine
Catalog No.:BCN5076
CAS No.:22864-92-2
- Anisomycin
Catalog No.:BCC7007
CAS No.:22862-76-6
- Ki8751
Catalog No.:BCC1116
CAS No.:228559-41-9
- VULM 1457
Catalog No.:BCC7533
CAS No.:228544-65-8
- 9-Epiblumenol B
Catalog No.:BCN5075
CAS No.:22841-42-5
- Pratensein
Catalog No.:BCN2918
CAS No.:2284-31-3
- Aspartame
Catalog No.:BCC8836
CAS No.:22839-47-0
- Boc-D-Val-OH
Catalog No.:BCC3466
CAS No.:22838-58-0
- Miconazole nitrate
Catalog No.:BCC9047
CAS No.:22832-87-7
- Hypoglaunine A
Catalog No.:BCN3086
CAS No.:228259-16-3
- Boc-Met-OH.DCHA
Catalog No.:BCC2602
CAS No.:22823-50-3
- Alisol K 23-acetate
Catalog No.:BCN3363
CAS No.:228095-18-9
- Silymarin
Catalog No.:BCN6299
CAS No.:22888-70-6
- 4-Amino-3,5-dichloropyridine
Catalog No.:BCC8679
CAS No.:22889-78-7
- TAK-779
Catalog No.:BCC4137
CAS No.:229005-80-5
- R 892
Catalog No.:BCC5992
CAS No.:229030-05-1
- Ginkgolic acid C15:1
Catalog No.:BCN2307
CAS No.:22910-60-7
- Neferine
Catalog No.:BCN6338
CAS No.:2292-16-2
- Desmethoxycentaureidin
Catalog No.:BCN5077
CAS No.:22934-99-2
- Abn-CBD
Catalog No.:BCC7011
CAS No.:22972-55-0
- GW9662
Catalog No.:BCC2260
CAS No.:22978-25-2
- AGN 194310
Catalog No.:BCC5416
CAS No.:229961-45-9
- Atazanavir sulfate (BMS-232632-05)
Catalog No.:BCC2114
CAS No.:229975-97-7
- MPTP hydrochloride
Catalog No.:BCC1778
CAS No.:23007-85-4
Famprofazone use can be misinterpreted as methamphetamine abuse.[Pubmed:20663288]
J Anal Toxicol. 2010 Jul-Aug;34(6):347-53.
Famprofazone, a major ingredient of Gewolen, is an analgesic that has been demonstrated to be metabolized to methamphetamine (MA) and amphetamine (AM) following administration. Therefore, a Famprofazone user may be interpreted as an illicit MA abuser in Taiwan because the user's urine tested positive for MA. The purpose of this study was to investigate whether the concentration of MA metabolized from a single dose of Gewolen users would offend the official controlled substance regulation and be identified as MA-positive. Subjects (n = 6) received 25 mg of Famprofazone and collected all urine specimens at certain timed intervals for 48 h after drug administration. The urine specimens were screened by immunoassay and then confirmed by gas chromatography-mass spectrometry (GC-MS). The highest concentration of amphetamines by immunoassay was 1954 ng/mL, and 18.8% of the urine specimens' amphetamines concentrations exceeded 500 ng/mL. The MA and AM concentrations by GC-MS analysis of these urine specimens ranged from 901 to 2670 ng/mL and 208 to 711 ng/mL, respectively. These urine specimens were interpreted as MA-positive (>or= 500 ng/mL MA and >or= 100 ng/mL AM), according to the official test methods of Taiwan. The MA positive results appeared within 2-34 h. It is therefore clearly possible to misinterpret the legitimate Famprofazone user as an MA abuser in Taiwan.
Famprofazone as the source of methamphetamine and amphetamine in urine specimen collected during sport competition.[Pubmed:17316255]
J Forensic Sci. 2007 Mar;52(2):479-86.
During a sport competition event in Taiwan, one urine specimen was found positive for both methamphetamine (2688 ng/mL) and amphetamine (462 ng/mL). The specimen donor claimed that she had taken Gewolen (a nonprescription drug manufactured in Taiwan) for treating abdominal pain and the medication was presented. Laboratory investigation confirmed that Gewolen contains Famprofazone, which is known to metabolize to methamphetamine and amphetamine and is included in the prohibited list of the World Anti-Doping Agency. Study on the excretion profiles of three volunteers ingesting 50 mg Famprofazone produced the following patterns similar to that observed in the case specimen: (a) the ratio of methamphetamine to amphetamine was approximately 6 to 1; (b) d- and l-enantiomers of both methamphetamine and amphetamine were present, while the amount of l-methamphetamine was 3-4-fold greater than its counterpart. The data suggested that Famprofazone (as the ingredient of Gewolen) was likely the source of the prohibited drugs found in the case specimen.
Metabolic profile of famprofazone following multidose administration.[Pubmed:15516292]
J Anal Toxicol. 2004 Sep;28(6):432-8.
One of the 14 different drugs known to be metabolized to methamphetamine and/or amphetamine is Famprofazone, a component in the multi-ingredient formulation Gewodin. Because of its conversion to methamphetamine and amphetamine, which can result in positive drug-testing results, the excretion pattern of these metabolites is critical for proper interpretation of drug-testing results. Multiple doses of Famprofazone were administered to healthy volunteers with no previous history of methamphetamine, amphetamine, or Famprofazone use. Following administration, urine samples were collected ad lib for nine days, and pH, specific gravity, and creatinine values were determined. To determine the methamphetamine and amphetamine excretion profile, samples were extracted, derivatized, and analyzed by gas chromatography-mass spectrometry (GC-MS). Peak concentrations of methamphetamine ranged from 5327 to 14,155 ng/mL and from 833 to 3555 ng/mL for amphetamine and were reached between 12:22 and 48:45 h post initial dose. There were 15-19 samples per subject that were positive under HHS testing guidelines, with the earliest at 03:37 h post initial dose and as late as 70:30 h post last dose. Methamphetamine and amphetamine were last detected (LOD > or = 5 ng/mL) up to 159 h and 153 h post last dose for methamphetamine and amphetamine, respectively. GC-MS was also used to determine the enantiomeric composition of methamphetamine and amphetamine. This analysis revealed both enantiomers were present in a predictable pattern.
Metabolic profile of amphetamine and methamphetamine following administration of the drug famprofazone.[Pubmed:14607003]
J Anal Toxicol. 2003 Oct;27(7):479-84.
There are a several drugs that lead to the production of methamphetamine and/or amphetamine in the body which are subsequently excreted in the urine. These drugs raise obvious concerns when interpreting positive amphetamine drug testing results. Famprofazone is an analgesic found in a multi-ingredient medication (Gewodin) used for pain relief. Two Gewodin tablets (50 mg of Famprofazone) were administered orally to healthy volunteers with no history of amphetamine, methamphetamine, or Famprofazone use. Following administration, urine samples were collected ad lib for up to six days, and pH, specific gravity, and creatinine values were determined. In order to determine the quantitative excretion profile of amphetamine and methamphetamine, samples were extracted using liquid-liquid extraction, derivatized with heptafluorobutyric anhydride, and analyzed by gas chromatography-mass spectrometry (GC-MS). The ions monitored were 91, 118, 240 for amphetamine and 254, 210, 118 for methamphetamine. Amphetamine-d(6) and methamphetamine-d(11) were used as internal standards. Peak concentrations for amphetamine ranged from 148 to 2271 ng/mL and for methamphetamine 615 to 7361 ng/mL. Concentrations of both compounds peaked between 3 and 7 h post-dose. Amphetamine and methamphetamine could be detected (limit of detection = 5 ng/mL) at 121 and 143 h post-dose, respectively. Using a cutoff of 500 ng/mL, all subjects had individual urine samples that tested positive. One subject had 14 samples above the cutoff with the last positive being detected over 48 h post-dose. The profile of methamphetamine and amphetamine enantiomers was also determined using liquid-liquid extraction, derivatization with N-trifluoroacetyl-l-prolyl chloride and analysis by GC-MS. Data showed the Famprofazone metabolites amphetamine and methamphetamine to be both d- and l-enantiomers. The proportion of l-methamphetamine exceeded that of its d-enantiomer from the first sample collected. Initially, the proportion was approximately 70% l-methamphetamine and this proportion increased over time. Amphetamine results showed l- and d-amphetamine were virtually the same in the early samples with the proportion of l-amphetamine increasing as time progressed. Forensic interpretation of drug testing results is a challenging critical part of forensic drug testing area because of the potential repercussions the results found may have on an individual's life. The finding of each enantiomers by itself differentiates Famprofazone use from the most commonly abused form of methamphetamine and all medicinal methamphetamine available in the U.S., which is either d-methamphetamine (prescription medication) or l-methamphetamine (Vicks inhaler). Coupling this information with the concentrations of amphetamine and methamphetamine helps to determine the potential for use of this drug.


