BMS-690514HER/EGFR inhibitor CAS# 859853-30-8 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 859853-30-8 | SDF | Download SDF |
| PubChem ID | 11349170 | Appearance | Powder |
| Formula | C19H24N6O2 | M.Wt | 368.44 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | DMSO : ≥ 25 mg/mL (67.86 mM) *"≥" means soluble, but saturation unknown. | ||
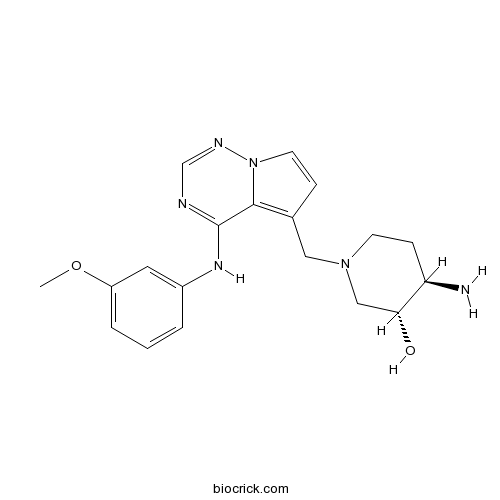
| Chemical Name | (3R,4R)-4-amino-1-[[4-(3-methoxyanilino)pyrrolo[2,1-f][1,2,4]triazin-5-yl]methyl]piperidin-3-ol | ||
| SMILES | COC1=CC=CC(=C1)NC2=NC=NN3C2=C(C=C3)CN4CCC(C(C4)O)N | ||
| Standard InChIKey | CSGQVNMSRKWUSH-IAGOWNOFSA-N | ||
| Standard InChI | InChI=1S/C19H24N6O2/c1-27-15-4-2-3-14(9-15)23-19-18-13(5-8-25(18)22-12-21-19)10-24-7-6-16(20)17(26)11-24/h2-5,8-9,12,16-17,26H,6-7,10-11,20H2,1H3,(H,21,22,23)/t16-,17-/m1/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | BMS-690514 is a potent and orally active inhibitor of EGFR and VEGFR; has IC50s of 5, 20 and 60 nM for EGFR, HER 2 and HER 4, respectively.In Vitro:BMS-690514 targets several critical signaling pathways: human epidermal growth factor receptor (HER)/ErbB, angiogenesis signaling through VEGFR2, lymphangiogenesis through VEGFR3, and also shows activity against VEGFR1, Flt-3, and Lck. Permeability of BMS-690514 in Caco-2 cells is in the intermediate range with a moderate potential to be a P-gp substrate[2]. BMS-690514 inhibits members of the VEGFR family with IC50 values in the range of 25 to 50 nM. Non–small cell lung tumor cells with exon 19 deletion (HCC4006, HCC827, and PC9) are highly sensitive to BMS-690514, which inhibits their proliferation with IC50 values of 2 to 35 nM. Tumor cell lines with EGFR gene amplification (DiFi, NCI-H2073, A431) are also highly sensitive to inhibition by BMS-690514. Tumor cell lines that are dependent on HER2 signaling are also found to be highly sensitive to BMS-690514. Breast and gastric tumor cell lines that have HER2 gene amplification (N87, SNU-216, AU565, BT474, KPL4, and HCC202) are inhibited with IC50 values of 20 to 60 nM[1].In Vivo:BMS-690514 has been shown to be efficacious in a broad spectrum of tumor xenografts. At doses that are efficacious and well tolerated in the animal models, BMS-690514 inhibits tumor cell proliferation and tumor blood flow[1]. The oral bioavailability of BMS-690514 is 78% in mice, 100% in rats, 8% in monkeys, and 29% in dogs. BMS-690514 is able to cross the blood–brain barrier with a brain-to-plasma ratio of 1. The preclinical ADME properties of BMS-690514 suggest good oral bioavailability in humans and metabolism by multiple pathways including oxidation and glucuronidation[2]. References: | |||||

BMS-690514 Dilution Calculator

BMS-690514 Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.7141 mL | 13.5707 mL | 27.1415 mL | 54.2829 mL | 67.8537 mL |
| 5 mM | 0.5428 mL | 2.7141 mL | 5.4283 mL | 10.8566 mL | 13.5707 mL |
| 10 mM | 0.2714 mL | 1.3571 mL | 2.7141 mL | 5.4283 mL | 6.7854 mL |
| 50 mM | 0.0543 mL | 0.2714 mL | 0.5428 mL | 1.0857 mL | 1.3571 mL |
| 100 mM | 0.0271 mL | 0.1357 mL | 0.2714 mL | 0.5428 mL | 0.6785 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
BMS-690514, a potent inhibitor of human epidermal growth factor receptor (HER/EGFR). In addition to an improved potency in inhibiting HER1/HER2 with IC50 value of 5 and 19 nM, respectively, BMS-690514 also shows significant potency against other protein kinases, such as VEGFR2, Flt-3, and Lck with IC50 value of 50, 110, and 220 nM, respectively.[1]
The epidermal growth factor receptor is a member of the ErbB family of receptors, EGFR (ErbB-1), HER2/c-neu (ErbB-2), Her 3 (ErbB-3) and Her 4 (ErbB-4) [2]. Upon activation by its growth factor ligands, EGFR undergoes a transition from an inactive monomeric form to an active homodimer[3]. In addition to forming homodimers after ligand binding, EGFR may pair with another member of the ErbB receptor family, such as ErbB2/Her2/neu, to create an activated heterodimer. EGFR dimerization stimulates its intrinsic intracellular protein-tyrosine kinase activity, resulting autophosphorylation[4], which elicits downstream activation and signaling by several other proteins with SH2 domains that can initiate several signal transduction cascades, principally the MAPK, Akt and JNK pathways, leading to DNA synthesis and cell proliferation.[5]
Mutations affecting EGFR expression or activity could result in cancer.[6] BMS-690514 is an oral oncologic agent being developed for the treatment of patients with advanced nonsmall cell lung cancer and other solid tumor, for inhibiting the EGFR tyrosine kinase, which is on the cytoplasmic side of the receptor. Without kinase activity, EGFR is unable to activate itself, which is a prerequisite for binding of downstream adaptor proteins. Ostensibly by halting the signaling cascade in cells that rely on this pathway for growth, tumor proliferation and migration is diminished. BMS-690514 is metabolized via multiple metabolic pathways, including P450-mediated oxidation at one of the carbons of its pyrrolotriazine group at this site results in the formation of two metabolites, M1 and M37, through HPLC, NMR, LC/MS/MS and radiochromatographic analysis[7].
References:
1.Punit Marathe. et al. Preclinical Pharmacokinetics and In Vitro Metabolism of BMS-690514, a Potent Inhibitor of EGFR and VEGFR2. Journal of Pharmaceutical Sciences. 2010, 99(8),3579–3593.
2.Herbst RS."Review of epidermal growth factor receptor biology". Int. J. Radiat. Oncol. Biol. Phys.2004, 59 (2 Suppl): 21–6.
3.Yosef Yarden and Joseph Schlessinger. "Epidermal Growth-Factor Induces Rapid, Reversible Aggregation of the Purified Epidermal Growth-Factor Receptor". Biochemistry 1987, 26 (5): 1443–1451.
4.Downward J, Parker P, Waterfield MD. "Autophosphorylation sites on the epidermal growth factor receptor". Nature 1984, 311 (5985): 483–5.
5.Oda K, Matsuoka Y, Funahashi A, Kitano H. "A comprehensive pathway map of epidermal growth factor receptor signaling". Mol. Syst. Biol. 2005, 1 (1): 2005.0010.
6.Zhang H, Berezov A, Wang Q, Zhang G, Drebin J, Murali R, Greene MI. "ErbB receptors: from oncogenes to targeted cancer therapies". J. Clin. Invest. 2007, 117 (8): 2051–8.
7.Haizheng Hong. et al. Mechanistic Studies on a P450-Mediated Rearrangement of BMS-690514: Conversion of a Pyrrolotriazine to a Hydroxypyridotriazine.Chem. Res. Toxicol. 2011, 24, 125–134.
- FIT
Catalog No.:BCC7082
CAS No.:85951-63-9
- GR 125487 sulfamate
Catalog No.:BCC7142
CAS No.:859502-43-5
- AGN 205728
Catalog No.:BCC5418
CAS No.:859498-05-8
- Acetyl meldrum's acid
Catalog No.:BCC8805
CAS No.:85920-63-4
- 4-O-Methylhonokiol
Catalog No.:BCN8474
CAS No.:68592-15-4
- 6-Iodonordihydrocapsaicin
Catalog No.:BCC5860
CAS No.:859171-97-4
- Lincomycin hydrochloride
Catalog No.:BCC9011
CAS No.:859-18-7
- prim-O-Glucosylangelicain
Catalog No.:BCN4409
CAS No.:85889-15-2
- 1,3-Dipropyl-8-phenylxanthine
Catalog No.:BCC6664
CAS No.:85872-53-3
- Marsdenoside F
Catalog No.:BCN4564
CAS No.:858360-61-9
- Vialinin A
Catalog No.:BCC2367
CAS No.:858134-23-3
- 3-O-Benzyl estrone
Catalog No.:BCC8638
CAS No.:858-98-0
- Anemosapogenin
Catalog No.:BCN2454
CAS No.:85999-40-2
- Carbazole
Catalog No.:BCN6903
CAS No.:86-74-8
- Benzoyleneurea
Catalog No.:BCC8865
CAS No.:86-96-4
- Cyclovirobuxine
Catalog No.:BCN5965
CAS No.:860-79-7
- AZD7762
Catalog No.:BCC2555
CAS No.:860352-01-8
- Fmoc-Cys(Acm)-OH
Catalog No.:BCC3473
CAS No.:86060-81-3
- Fmoc-Lys(Z)-OH
Catalog No.:BCC3525
CAS No.:86060-82-4
- Fmoc-Asp-OBzl
Catalog No.:BCC3087
CAS No.:86060-83-5
- Fmoc-Gly-OPfp
Catalog No.:BCC3499
CAS No.:86060-85-7
- Fmoc-Ala-OPfp
Catalog No.:BCC3035
CAS No.:86060-86-8
- Fmoc-Val-OPfp
Catalog No.:BCC3571
CAS No.:86060-87-9
- Fmoc-Leu-OPfp
Catalog No.:BCC3510
CAS No.:86060-88-0
Inhibitory effect of ketoconazole on the pharmacokinetics of a multireceptor tyrosine kinase inhibitor BMS-690514 in healthy participants: assessing the mechanism of the interaction with physiologically-based pharmacokinetic simulations.[Pubmed:23436267]
J Clin Pharmacol. 2013 Feb;53(2):217-27.
BMS-690514, a selective inhibitor of the ErbB and vascular endothelial growth factor receptors, has shown antitumor activity in early clinical development. The compound is metabolized by multiple enzymes, with CYP3A4 responsible for the largest fraction (34%) of metabolism. It is also a substrate of P-glycoprotein (P-gp) in vitro. To assess the effect of ketoconazole on BMS-690514 pharmacokinetics, 17 healthy volunteers received 200 mg BMS-690514 alone followed by 100 mg BMS-690514 with ketoconazole (400 mg once daily for 4 days). The AUC(infinity) of 100 mg BMS-690514 concomitantly administered with ketoconazole was similar to that of 200 mg BMS-690514 alone. The dose-normalized C(max) and AUC(infinity) of BMS-690514 from the 100-mg BMS-690514/400-mg ketoconazole treatment increased by 55% and 127%, respectively, relative to those from 200 mg BMS-690514 alone. Prediction of the drug-drug interaction (DDI) using a population-based simulator (Simcyp) indicated that, in addition to CYP3A4 inhibition, the inhibition of P-gp by ketoconazole in the intestine, liver, and kidneys must be invoked to fully account for the DDI observed. This finding suggests that the inhibition of P-gp by ketoconazole, along with its effect on CYP3A4, needs to be considered when designing a DDI study of ketoconazole with a victim drug that is a dual substrate.
A phase I study of BMS-690514 in Japanese patients with advanced or metastatic solid tumors.[Pubmed:22878519]
Cancer Chemother Pharmacol. 2012 Oct;70(4):559-65.
PURPOSE: BMS-690514 is a novel oral tyrosine kinase inhibitor of ErbB and vascular endothelial growth factor receptor. This open-label phase I dose-escalation study (ClinicalTrials.gov Identifier: NCT00516451) aimed to assess the safety, preliminary efficacy, pharmacokinetics, and pharmacodynamics of BMS-690514 in Japanese patients with advanced or metastatic solid tumors. METHODS: Patients with advanced or metastatic solid tumors received oral BMS-690514 once daily continuously until disease progression or intolerable toxicity occurred. Dose-limiting toxicity (DLT) was evaluated from the first dose to Day 29. Dose levels at 100 and 200 mg were investigated. Assessments included adverse events, tumor response, pharmacokinetics, pharmacodynamics, 2 [18F] fluoro-2-deoxyglucose positron-emitting tomography, and epidermal growth factor receptor and K-ras mutations. RESULTS: BMS-690514 at the dose of 100 mg (n = 3) or 200 mg (n = 3) was administered once daily to totally nine patients and was well tolerated up to 200 mg. No treatment-related serious adverse events or DLTs were reported. Frequently observed treatment-related AEs were acne, diarrhea, dry skin, hypertension, stomatitis, blood fibrinogen increased, hemoglobin decreased, pruritus, and hypoalbuminemia. These were generally reported as Grade 1 and 2. Five of 9 patients (56 %) had stable disease. Plasma concentrations of BMS-690514 reached Cmax within 3 h and declined with an effective half-life of approximately 10 and 12 h at 100 and 200 mg, respectively. CONCLUSIONS: Oral BMS-690514 was well tolerated in Japanese patients with advanced or metastatic solid tumors up to 200 mg.
A phase I trial to determine the safety, pharmacokinetics, and pharmacodynamics of intercalated BMS-690514 with paclitaxel/carboplatin (PC) in advanced or metastatic solid malignancies.[Pubmed:23468081]
Cancer Chemother Pharmacol. 2013 May;71(5):1273-85.
PURPOSE: A phase I dose escalation study was performed to determine the maximum tolerated dose (MTD) of intercalated dosing of BMS-690514, a reversible oral panHER/VEGF receptor inhibitor, combined with paclitaxel/carboplatin (PC) in advanced solid tumors. Secondary endpoints included safety, pharmacokinetics (PK), exploratory pharmacodynamics (PD), and preliminary efficacy. EXPERIMENTAL DESIGN: Patients received fixed doses of P (200 mg/m(2)) and C (AUC 6 mg/mL min) q21 days with intercalated BMS-690514 (Days 4-19) starting at 100 mg/day and increasing by 50 mg/day using a 3 + 3 dose escalation design until the MTD was reached. Twenty additional patients were enrolled in the expansion cohort at the recommended phase II dose (RP2D). RESULTS: The MTD was reached at 150 mg/day. DLTs included grade 3 thrombosis at 100 mg (1 patient) and grade 3 diarrhea at 150 mg (1 patient) and 200 mg (2 patients). Serious adverse events (AEs) occurring in 20/37 patients included neutropenia (n = 5), diarrhea (n = 4), pulmonary embolism (n = 3), and simultaneous dehydration, acute renal failure, and febrile neutropenia (n = 2). BMS-690514-related AEs included diarrhea (97 %), acneiform rash (60 %), fatigue (43 %), nausea (30 %), and anorexia (30 %). There were no treatment-related deaths. Sequential intermittent administration of PC did not affect the PK of BMS-690514. Of the 32 patients evaluable for efficacy, there were 12 partial responses including five patients with non-small-cell lung cancer and 12 patients with stable disease. CONCLUSIONS: The MTD of intercalated BMS-609514 combined with PC was 150 mg/day. This approach was tolerable with manageable toxicities and antitumor activity in a variety of solid tumor types.


