BetonicineCAS# 515-25-3 |
- (+)-Turicine
Catalog No.:BCC8361
CAS No.:515-24-2
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 515-25-3 | SDF | File under preparation. |
| PubChem ID | 164642 | Appearance | Powder |
| Formula | C7H13NO3 | M.Wt | 159.2 |
| Type of Compound | Alkaloids | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
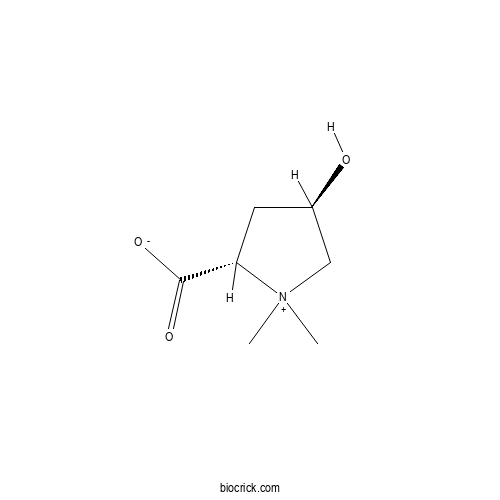
| Chemical Name | (2S,4R)-4-hydroxy-1,1-dimethylpyrrolidin-1-ium-2-carboxylate | ||
| SMILES | C[N+]1(CC(CC1C(=O)[O-])O)C | ||
| Standard InChIKey | MUNWAHDYFVYIKH-RITPCOANSA-N | ||
| Standard InChI | InChI=1S/C7H13NO3/c1-8(2)4-5(9)3-6(8)7(10)11/h5-6,9H,3-4H2,1-2H3/t5-,6+/m1/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Betonicine will be useful for elucidating the interaction between OHL and TraR, and for developing novel bacterial quorum-sensing inhibitors. | |||||

Betonicine Dilution Calculator

Betonicine Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 6.2814 mL | 31.407 mL | 62.8141 mL | 125.6281 mL | 157.0352 mL |
| 5 mM | 1.2563 mL | 6.2814 mL | 12.5628 mL | 25.1256 mL | 31.407 mL |
| 10 mM | 0.6281 mL | 3.1407 mL | 6.2814 mL | 12.5628 mL | 15.7035 mL |
| 50 mM | 0.1256 mL | 0.6281 mL | 1.2563 mL | 2.5126 mL | 3.1407 mL |
| 100 mM | 0.0628 mL | 0.3141 mL | 0.6281 mL | 1.2563 mL | 1.5704 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- 1-(2-Hydroxyphenyl)-3-phenyl-2-propenone
Catalog No.:BCN9742
CAS No.:1214-47-7
- Quercetin 3,4'-diglucoside
Catalog No.:BCN9741
CAS No.:29125-80-2
- Clausine E
Catalog No.:BCN9740
CAS No.:182261-83-2
- Ethyl 3-hydroxybenzoate
Catalog No.:BCN9739
CAS No.:7781-98-8
- Uzarin
Catalog No.:BCN9738
CAS No.:20231-81-6
- Capsanthin
Catalog No.:BCN9737
CAS No.:465-42-9
- Tripalmitin
Catalog No.:BCN9736
CAS No.:555-44-2
- 1-(3',5'-dimethoxy)phenyl-2-[4''-O-beta-D-glucopyranosyl (6->1)-O-alpha-L-rhamnopyranosyl]phenylethane
Catalog No.:BCN9735
CAS No.:1338076-61-1
- gamma-Nonanolactone
Catalog No.:BCN9734
CAS No.:104-61-0
- Furfuryl alcohol
Catalog No.:BCN9733
CAS No.:98-00-0
- Carvacryl acetate
Catalog No.:BCN9732
CAS No.:6380-28-5
- Rauhimbine
Catalog No.:BCN9731
CAS No.:66634-44-4
- (+)-atechin 5-gallate
Catalog No.:BCN9744
CAS No.:128232-62-2
- Hibifolin
Catalog No.:BCN9745
CAS No.:55366-56-8
- 2-Naphthol
Catalog No.:BCN9746
CAS No.:135-19-3
- 7-Methoxyflavonol
Catalog No.:BCN9747
CAS No.:7478-60-6
- Homoembelin
Catalog No.:BCN9748
CAS No.:38363-99-4
- Neosalvianen
Catalog No.:BCN9749
CAS No.:790673-00-6
- Artemicapin C
Catalog No.:BCN9750
CAS No.:334007-19-1
- 3',4',5,5',6,7-Hexamethoxyflavone
Catalog No.:BCN9751
CAS No.:29043-07-0
- Benzyl acetate
Catalog No.:BCN9752
CAS No.:140-11-4
- (+)-Menthofuran
Catalog No.:BCN9753
CAS No.:17957-94-7
- Arganine B
Catalog No.:BCN9754
CAS No.:144425-21-8
- 5-Methoxyflavanone
Catalog No.:BCN9755
CAS No.:55947-36-9
Co-expression network analysis reveals the pivotal role of mitochondrial dysfunction and interferon signature in juvenile dermatomyositis.[Pubmed:32110496]
PeerJ. 2020 Feb 18;8:e8611.
Background: Juvenile dermatomyositis (JDM) is an immune-mediated disease characterized by chronic organ inflammation. The pathogenic mechanisms remain ill-defined. Methods: Raw microarray data of JDM were obtained from the gene expression omnibus (GEO) database. Based on the GSE3307 dataset with 39 samples, weighted correlation network analysis (WGCNA) was performed to identify key modules associated with pathological state. Functional enrichment analyses were conducted to identify potential mechanisms. Based on the criteria of high connectivity and module membership, candidate hub genes were selected. A protein-protein interaction network was constructed to identify hub genes. Another dataset (GSE11971) was used for the validation of real hub genes. Finally, the real hub genes were used to screen out small-molecule compounds via the Connectivity map database. Results: Three modules were considered as key modules for the pathological state of JDM. Functional enrichment analysis indicated that responses to interferon and metabolism were dysregulated. A total of 45 candidate hub genes were selected according to the pre-established criteria, and 20 genes could differentiate JDM from normal controls by validation of another external dataset (GSE11971). These real hub genes suggested the pivotal role of mitochondrial dysfunction and interferon signature in JDM. Furthermore, drug repositioning highlighted the importance of acacetin, helveticoside, lanatoside C, deferoxamine, LY-294002, tanespimycin and L01AD from downregulated genes with the potential to perturb the development of JDM, while Betonicine, felodipine, valproic acid, trichostatin A and sirolimus from upregulated genes provided potentially therapeutic goals for JDM. Conclusions: There are 20 real hub genes associated with the pathological state of JDM, suggesting the pivotal role of mitochondrial dysfunction and interferon signature in JDM. This analysis predicted several kinds of small-molecule compounds to treat JDM.
Sinorhizobium meliloti chemotaxis to quaternary ammonium compounds is mediated by the chemoreceptor McpX.[Pubmed:27748981]
Mol Microbiol. 2017 Jan;103(2):333-346.
The bacterium Sinorhizobium meliloti is attracted to seed exudates of its host plant alfalfa (Medicago sativa). Since quaternary ammonium compounds (QACs) are exuded by germinating seeds, we assayed chemotaxis of S. meliloti towards Betonicine, choline, glycine betaine, stachydrine and trigonelline. The wild type displayed a positive response to all QACs. Using LC-MS, we determined that each germinating alfalfa seed exuded QACs in the nanogram range. Compared to the closely related nonhost species, spotted medic (Medicago arabica), unique profiles were released. Further assessments of single chemoreceptor deletion strains revealed that an mcpX deletion strain displayed little to no response to these compounds. Differential scanning fluorimetry showed interaction of the isolated periplasmic region of McpX (McpX(PR) and McpX34-306 ) with QACs. Isothermal titration calorimetry experiments revealed tight binding to McpX(PR) with dissociation constants (Kd ) in the nanomolar range for choline and glycine betaine, micromolar Kd for stachydrine and trigonelline and a Kd in the millimolar range for Betonicine. Our discovery of S. meliloti chemotaxis to plant-derived QACs adds another role to this group of compounds, which are known to serve as nutrient sources, osmoprotectants and cell-to-cell signalling molecules. This is the first report of a chemoreceptor that mediates QACs taxis through direct binding.
A serum metabolomics-driven approach predicts orange juice consumption and its impact on oxidative stress and inflammation in subjects from the BIONAOS study.[Pubmed:27689343]
Mol Nutr Food Res. 2017 Feb;61(2).
SCOPE: To identify biomarkers of orange juice (OJ) consumption containing different doses of polyphenols and to determine its impact on oxidative stress and inflammation using an untargeted metabolomics analysis. METHODS AND RESULTS: Thirty subjects aged 22-63 years from the BIONAOS study consumed a normal-polyphenol OJ (NPJ) or a high-polyphenol OJ (HPJ) (299 or 745 mg/L, respectively) for 12 weeks in a randomized, parallel, double-blind study. UHPLC-MS, univariate and multivariate statistical analysis and ROC curves were used to design biomarkers of consumption in serum. We propose Betonicine, stachydrine, methyl glucopyranoside (alpha+beta), dihydroferulic acid and galactonate as a new metabolic signature to distinguish the intake of OJ with a different polyphenol content. Changes in metabolites related to OJ, oxidative stress and inflammation were observed. After HPJ consumption, the serum levels of hydroxyoctadecadienoic acid (9-HODE+13-HODE) and dihydroxyoctadecanoic acid (12,13-DiHOME and 9,10-DiHOME) decreased, whereas levels of 12-hydroxyeicosatetraenoic acid (12-HETE) increased. 5-HETE increased after the NPJ intervention exclusively. CONCLUSION: We designed a new panel of biomarkers to differentiate the intake of OJs containing different doses of polyphenols. On the other hand, the consumption of an OJ with a high content of flavanones improved oxidative stress and inflammatory biomarkers.
Plant-derived compatible solutes proline betaine and betonicine confer enhanced osmotic and temperature stress tolerance to Bacillus subtilis.[Pubmed:25012968]
Microbiology (Reading). 2014 Oct;160(Pt 10):2283-2294.
L-Proline is a widely used compatible solute and is employed by Bacillus subtilis, through both synthesis and uptake, as an osmostress protectant. Here, we assessed the stress-protective potential of the plant-derived L-proline derivatives N-methyl-L-proline, L-proline betaine (stachydrine), trans-4-L-hydroxproline and trans-4-hydroxy-L-proline betaine (Betonicine) for cells challenged by high salinity or extremes in growth temperature. l-Proline betaine and Betonicine conferred salt stress protection, but trans-4-L-hydroxyproline and N-methyl-L-proline was unable to do so. Except for L-proline, none of these compounds served as a nutrient for B. subtilis. L-Proline betaine was a considerably better osmostress protectant than Betonicine, and its import strongly reduced the l-proline pool produced by B. subtilis under osmotic stress conditions, whereas a supply of Betonicine affected the L-proline pool only modestly. Both compounds downregulated the transcription of the osmotically inducible opuA operon, albeit to different extents. Mutant studies revealed that L-proline betaine was taken up via the ATP-binding cassette transporters OpuA and OpuC, and the betaine-choline-carnitine-transporter-type carrier OpuD; Betonicine was imported only through OpuA and OpuC. L-Proline betaine and Betonicine also served as temperature stress protectants. A striking difference between these chemically closely related compounds was observed: L-proline betaine was an excellent cold stress protectant, but did not provide heat stress protection, whereas the reverse was true for Betonicine. Both compounds were primarily imported in temperature-challenged cells via the high-capacity OpuA transporter. We developed an in silico model for the OpuAC-Betonicine complex based on the crystal structure of the OpuAC solute receptor complexed with L-proline betaine.
Betaines in fruits of Citrus genus plants.[Pubmed:21838291]
J Agric Food Chem. 2011 Sep 14;59(17):9410-6.
Numerous compounds, many of them osmolytes, were quantified in natural juices and in frozen concentrate juices from fruits of plants of the Citrus genus. L-proline, N-methyl-L-proline (hygric acid), N,N-dimethyl-L-proline (stachydrine), 4-hydroxy-L-prolinebetaine (Betonicine), 4-hydroxy-L-proline, gamma-aminobutyric acid (Gaba), 3-carboxypropyltrimethylammonium (GabaBet), N-methylnicotinic acid (trigonelline), and choline in the fruit juices of yellow orange, blood orange, lemon, mandarin, bitter orange (Citrus aurantium), chinotto (Citrus myrtifolia), and grapefruit were analyzed by sensitive HPLC-ESI-tandem mass spectrometry procedure. It was found that the most represented osmolytes in the juices, that is, L-proline, stachydrine, and Betonicine, can be quantified with minimal sample preparation and short analysis time (about 1 min) also by flow injection analysis (FIA) ESI-MS/MS with the same results as obtained by HPLC ESI-MS/MS. In all of the juices, discrete amounts of choline and trigonelline were present. Conversely, GabaBet was always below detection limits. Notably, N-methyl-L-proline and 4-hydroxy-L-prolinebetaine, which were discovered for the first time in the juice of bergamot (Citrus bergamia Risso et Poit), are also present in all of the citrus juices examined.


