HibifolinCAS# 55366-56-8 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 55366-56-8 | SDF | Download SDF |
| PubChem ID | 5490334 | Appearance | Yellow powder |
| Formula | C21H18O14 | M.Wt | 494.4 |
| Type of Compound | Flavonoids | Storage | Desiccate at -20°C |
| Synonyms | Gossypetin 8-glucuronide; 3,3',4',5,7,8-Hexahydroxyflavone 8-glucuronide | ||
| Solubility | Soluble in methan | ||
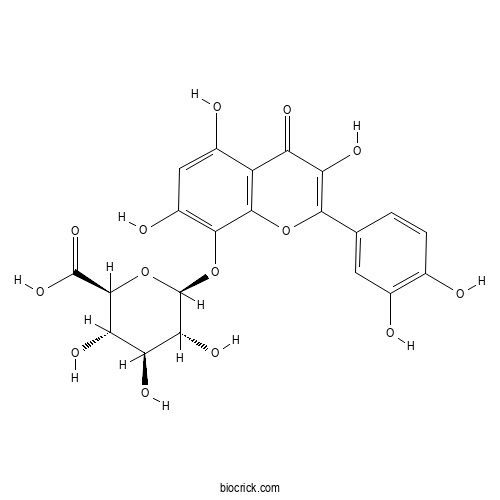
| Chemical Name | (2S,3S,4S,5R,6S)-6-[2-(3,4-dihydroxyphenyl)-3,5,7-trihydroxy-4-oxochromen-8-yl]oxy-3,4,5-trihydroxyoxane-2-carboxylic acid | ||
| SMILES | C1=CC(=C(C=C1C2=C(C(=O)C3=C(O2)C(=C(C=C3O)O)OC4C(C(C(C(O4)C(=O)O)O)O)O)O)O)O | ||
| Standard InChIKey | KHVMAMXQPVHXTJ-ORYXKJSJSA-N | ||
| Standard InChI | InChI=1S/C21H18O14/c22-6-2-1-5(3-7(6)23)16-13(28)11(26)10-8(24)4-9(25)17(18(10)33-16)34-21-15(30)12(27)14(29)19(35-21)20(31)32/h1-4,12,14-15,19,21-25,27-30H,(H,31,32)/t12-,14-,15+,19-,21+/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Hibifolin can act as a potential inhibitor of ADA, it protects neurons against A beta-induced apoptosis and stimulates Akt activation, which would be useful in developing potential drugs or food supplements for treating AD. Hibifolin behaves as a dual inhibitor of PGE2 and leukotriene B4 (LTB4) formation in peritoneal exudates, thus it shows anti-inflammatory activity. | |||||

Hibifolin Dilution Calculator

Hibifolin Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.0227 mL | 10.1133 mL | 20.2265 mL | 40.4531 mL | 50.5663 mL |
| 5 mM | 0.4045 mL | 2.0227 mL | 4.0453 mL | 8.0906 mL | 10.1133 mL |
| 10 mM | 0.2023 mL | 1.0113 mL | 2.0227 mL | 4.0453 mL | 5.0566 mL |
| 50 mM | 0.0405 mL | 0.2023 mL | 0.4045 mL | 0.8091 mL | 1.0113 mL |
| 100 mM | 0.0202 mL | 0.1011 mL | 0.2023 mL | 0.4045 mL | 0.5057 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- (+)-atechin 5-gallate
Catalog No.:BCN9744
CAS No.:128232-62-2
- Betonicine
Catalog No.:BCN9743
CAS No.:515-25-3
- 1-(2-Hydroxyphenyl)-3-phenyl-2-propenone
Catalog No.:BCN9742
CAS No.:1214-47-7
- Quercetin 3,4'-diglucoside
Catalog No.:BCN9741
CAS No.:29125-80-2
- Clausine E
Catalog No.:BCN9740
CAS No.:182261-83-2
- Ethyl 3-hydroxybenzoate
Catalog No.:BCN9739
CAS No.:7781-98-8
- Uzarin
Catalog No.:BCN9738
CAS No.:20231-81-6
- Capsanthin
Catalog No.:BCN9737
CAS No.:465-42-9
- Tripalmitin
Catalog No.:BCN9736
CAS No.:555-44-2
- 1-(3',5'-dimethoxy)phenyl-2-[4''-O-beta-D-glucopyranosyl (6->1)-O-alpha-L-rhamnopyranosyl]phenylethane
Catalog No.:BCN9735
CAS No.:1338076-61-1
- gamma-Nonanolactone
Catalog No.:BCN9734
CAS No.:104-61-0
- Furfuryl alcohol
Catalog No.:BCN9733
CAS No.:98-00-0
- 2-Naphthol
Catalog No.:BCN9746
CAS No.:135-19-3
- 7-Methoxyflavonol
Catalog No.:BCN9747
CAS No.:7478-60-6
- Homoembelin
Catalog No.:BCN9748
CAS No.:38363-99-4
- Neosalvianen
Catalog No.:BCN9749
CAS No.:790673-00-6
- Artemicapin C
Catalog No.:BCN9750
CAS No.:334007-19-1
- 3',4',5,5',6,7-Hexamethoxyflavone
Catalog No.:BCN9751
CAS No.:29043-07-0
- Benzyl acetate
Catalog No.:BCN9752
CAS No.:140-11-4
- (+)-Menthofuran
Catalog No.:BCN9753
CAS No.:17957-94-7
- Arganine B
Catalog No.:BCN9754
CAS No.:144425-21-8
- 5-Methoxyflavanone
Catalog No.:BCN9755
CAS No.:55947-36-9
- 6,7-Dihydroxyflavone
Catalog No.:BCN9756
CAS No.:38183-04-9
- Octanoic acid
Catalog No.:BCN9757
CAS No.:124-07-2
Inhibitory activity of hibifolin on adenosine deaminase- experimental and molecular modeling study.[Pubmed:27591790]
Comput Biol Chem. 2016 Oct;64:353-358.
Adenosine deaminase (ADA) is an enzyme involved in purine metabolism. ADA converts adenosine to inosine and liberates ammonia. Because of their critical role in the differentiation and maturation of cells, the regulation of ADA activity is considered as a potential therapeutic approach to prevent malignant and inflammatory disorders. In the present study, the inhibitory activity of a plant flavonoid, Hibifolin on ADA is investigated using enzyme kinetic assay and isothermal titration calorimetry. The inhibitory constant of Hibifolin was found to be 49.92muM+/-3.98 and the mode of binding was reversible. Isothermal titration calorimetry showed that the compound binds ADA with binding energy of -7.21Kcal/mol. The in silico modeling and docking studies showed that the bound ligand is stabilized by hydrogen bonds with active site residues of the enzyme. The study reveals that Hibifolin can act as a potential inhibitor of ADA.
Extraction of Flavonoids from the Flowers of Abelmoschus manihot (L.) Medic by Modified Supercritical CO(2) Extraction and Determination of Antioxidant and Anti-Adipogenic Activity.[Pubmed:27347916]
Molecules. 2016 Jun 25;21(7). pii: molecules21070810.
Abelmoschus manihot (L.) Medic has been used for many years in Chinese traditional medicine. In this study, supercritical CO(2) plus a modifier was utilized to extract flavonoids from the flowers of Abelmoschus manihot (L.) Medic. The effects of temperature (40 degrees C-60 degrees C), pressure (10-30 MPa) and different concentrations of ethanol as modifier (60%-90%, ethanol:water, v/v) on major flavonol content and the antioxidant activity of the extracts were studied by response surface methodology (RSM) using a Box-Behnken design. The flavonol content was calculated as the sum of the concentrations of seven major flavonoids, namely rutin, hyperin, isoquercetin, Hibifolin, myricetin, quercetin-3'-O-glucoside and quercetin, which were simultaneously determined by a HPLC method. The antioxidant activity was evaluated by a 2,2-diphenyl-1-picrylhydarzyl (DPPH) free radical-scavenging assay. The results showed that three factors and their interactions could be well fitted to second-order polynomial models (p < 0.05). At the optimal extraction conditions for flavonol content (20 MPa, 52 degrees C, and 85% ethanol content), the yield of flavonoids was 41.96 mg/g and the IC50 value was 0.288 mg/mL, respectively, suggesting the extract has high antioxidant activity. Furthermore, the anti-adipogenic activity of the extract on the 3T3-L1 cell line was investigated. The results indicated that it can downregulate PPARgamma and C/EBPalpha expression at mRNA. In summary, in this study, we have established a cost-effective method for the extraction of flavonoids from the flowers of Abelmoschus manihot (L.) Medic using supercritical fluid extraction and the extracts exhibited potent antioxidant and anti-adipogenic effects, suggesting a possible therapeutic approach for the prevention and treatment of obesity.
Abelmoschi Corolla non-flavonoid components altered the pharmacokinetic profile of its flavonoids in rat.[Pubmed:23702043]
J Ethnopharmacol. 2013 Jul 30;148(3):804-11.
AIM: Abelmoschi Corolla is a well-known herbal medicine used for the treatment of chronic renal disease. Flavonoids are the major bioactive ingredients of Abelmoschi Corolla, but some non-flavonoid components also exist in this herb. In order to clarify the influences of non-flavonoid components on the pharmacokinetics profile of the flavonoid fraction from Abelmoschi Corolla (FFA), an investigation was carried out to compare the pharmacokinetic parameters of seven flavonoid components after administration of FFA and after administration of FFA combined with different non-flavonoid fractions. MATERIALS AND METHODS: A selective and sensitive UPLC-MS/MS method was established to determine the plasma concentrations of the seven compounds. Sprague-Dawley rats were allocated to four groups which orally administered FFA, FFA combined with macromolecular fraction (FFA-MF), FFA combined with small molecule fraction (FFA-SF) and FFA combined with MF-SF (FFA-MF-SF) with approximately the same dose of FFA. At different time points, the concentration of rutin (1), hyperoside (2), isoquercitrin (3), Hibifolin (4), myricetin (5), quercetin-3'-O-glucose (6), quercetin (7) in rat plasma were determined and main pharmacokinetic parameters including T(1/2), T(max), AUC and C(max) were calculated using the DAS 2.0 software package. The statistical analysis was performed using the Student's t-test with P<0.05 as the level of significance. RESULTS: Flavonoids almost had similar pharmacokinetics profile that were rapidly absorbed, reached the peak concentration at 30-60 min in group A, but the pharmacokinetic profiles and parameters of these flavonoids changed when co-administered with non-flavonoid components. It was found that AUC of five flavonoids but not Hibifolin and quercetin in group FFA-SF and group FFA-MF-SF increased (P<0.05) in comparison with group FFA while the tendency was not observed in group FFA-MF. Moreover, seven flavonoids had varying degrees of differences in the pharmacokinetics parameters such as C(max), T(max) and T(1/2) (P<0.05) in group FFA-MF, FFA-SF and FFA-MF-SF by comparison with group FFA. CONCLUSION: These results indicate that non-flavonoid components could improve the bioavailability and delay the elimination of some flavonoids in rat.
Anticonvulsant, antidepressant-like activity of Abelmoschus manihot ethanol extract and its potential active components in vivo.[Pubmed:21784623]
Phytomedicine. 2011 Nov 15;18(14):1250-4.
Depression is the most common psychiatric comorbidity in patients with epilepsy. Searching for antiepileptic (anticonvulsant) and antidepressant-like medicines from natural products is very important for the treatment of this disease. The flower of Abelmoschus manihot (Linn.) Medicus has been reported to have neuroprotective effect against cerebral ischemia injury. In order to further explore the activity of Abelmoschus manihot on the central nervous system, the anticonvulsant and antidepressant-like effects of Abelmoschus manihot ethanol extract (AMEE) as well as its potential active components in vivo was investigated in the present study. It was found that AMEE could protect mice against PTZ-induced clonic convulsions and mortality. AMEE could also decrease immobility time in the FST in mice. Furthermore, the potential active components of AMEE in rat brain were identified by ultra performance liquid chromatography-mass spectrometer (UPLC-MS). Five parent components including isoquercitrin, hyperoside, Hibifolin, quercetin-3'-O-glucoside, quercetin and three metabolites were detected in rat brain after administration of AMEE. In conclusion, eight flavonoids were identified in rat brain after administration of AMEE; meanwhile, these flavonoids might represent the potential bioactive components of AMEE and contribute to its anticonvulsant and antidepressant-like activity in vivo.
Identification of the potential active components of Abelmoschus manihot in rat blood and kidney tissue by microdialysis combined with ultra-performance liquid chromatography/quadrupole time-of-flight mass spectrometry.[Pubmed:21247814]
J Chromatogr B Analyt Technol Biomed Life Sci. 2011 Feb 15;879(5-6):317-25.
In this paper, microdialysis combining with ultra-performance liquid chromatography/quadrupole time-of-flight mass spectrometry (UPLC-Q-TOF/MS) was applied to simultaneously identify components in blood and kidney dialysis after oral administration of Abelmoschus manihot extract. Microdialysis probe was implanted in the jugular vein and the kidney medulla, respectively; microdialysis samples were collected continuously, transferred to microtubes and analyzed by UPLC-Q-TOF/MS. The components in microdialysis samples were separated by an UPLC HSS T3 column and eluted with acetonitrile and water (containing 0.1% formic acid) at a flow rate of 0.4 mL/min. The results showed that unbound constituents in blood circulation of the rat include hyperoside, isoquercitrin, quercetin monoglucuronide, quercetin-3'-O-glucoside, quercetin, myricetin, and Hibifolin while unbound constituents in kidney are hyperoside, isoquercitrin, quercetin monoglucuronide, which might be the potential active components in vivo. The developed method was simple and reliable, and could be adopted to rapidly screen and identify potential active components contributing to pharmacological effects of TCM and to better clarify its action mechanism.
Hibifolin, a flavonol glycoside, prevents beta-amyloid-induced neurotoxicity in cultured cortical neurons.[Pubmed:19539722]
Neurosci Lett. 2009 Sep 18;461(2):172-6.
The toxicity of aggregated beta-amyloid (A beta) has been implicated as a critical cause in the development of Alzheimer's disease (AD). Hibifolin, a flavonol glycoside derived from herbal plants, possessed a strong protective activity against cell death induced by aggregated A beta. Application of Hibifolin in primary cortical neurons prevented the A beta-induced cell death in a dose-dependent manner. In cultured cortical neurons, the pre-treatment of Hibifolin abolished A beta-induced Ca(2+) mobilization, and also reduced A beta-induced caspase-3 and caspase-7 activation. Moreover, DNA fragmentation induced by A beta could be suppressed by Hibifolin. In addition to such protection mechanisms, Hibifolin was able to induce Akt phosphorylation in cortical neurons, which could be another explanation for the neuroprotection activity. These results therefore provided the first evidence that Hibifolin protected neurons against A beta-induced apoptosis and stimulated Akt activation, which would be useful in developing potential drugs or food supplements for treating AD.
Simultaneous determination of seven active flavonols in the flowers of Abelmoschus manihot by HPLC.[Pubmed:19298707]
J Chromatogr Sci. 2009 Mar;47(3):206-10.
A high-performance liquid chromatography method is developed for the simultaneous quantification of seven flavonols, namely quercetin-3-O-robinobioside, hyperin, isoquercetin, Hibifolin, myricetin, quercetin-3'-O-glucoside, and quercetin, in the flower of Abelmoschus manihot. These seven flavonols are selected as chemical markers because they are the major pharmacologically active constituents in the flower. The method involves the use of a Thermo ODS-2HYEPRSIL reversed-phase column (5 microm, 250 x 4.6 mm) at 25 degrees C with a mixture of acetonitrile and aqueous H(3)PO(4) as the mobile phase and detection at 370 nm. The recovery of the method is 94.31-107.08% with an RSD < or = 3.14% and the linearity (r(2) > 0.9996) is obtained for all the flavonoids. The current assay method can be readily utilized for the determination of the flavonols present in the flower and is considered to be suitable for the quality control of A. manihot samples. The comparison of flowers collected from nine locations shows that flavonoid glucoside is more stable than aglycon in the flower. This is the first study that analyzes the stability of flavonoids in the flower of A. manihot. This research also provides important evidence that the flower is a potentially abundant resource for obtaining Hibifolin.
Metabolism of hibifolin by human intestinal bacteria.[Pubmed:19235125]
Planta Med. 2009 Apr;75(5):483-7.
Hibifolin, the highest-content bioactive flavonoid of the flowers of Abelmoschus manihot, was incubated with human intestinal bacteria, and four metabolites (1-4) were obtained from the incubated solution by chromatographic methods. The structures of the four metabolites were elucidated as gossypetin 8-O-beta-D-4''-deoxy- Delta(4'')-glucuropyranoside (1), gossypetin (2), quercetin (3), and 8-methoxy-quercetin (4), respectively, on the basis of UV, NMR, and MS data. Metabolite 1 was obtained as a new compound with a specific beta-D-4''-deoxy-Delta(4'')-glucuropyranosyl moiety, which was formed through a unique and novel metabolic pathway that has not been reported previously.
Flavonoids possess neuroprotective effects on cultured pheochromocytoma PC12 cells: a comparison of different flavonoids in activating estrogenic effect and in preventing beta-amyloid-induced cell death.[Pubmed:17323972]
J Agric Food Chem. 2007 Mar 21;55(6):2438-45.
Despite the classical hormonal effect, estrogen possesses a neuroprotective effect in the brain, which has led many to search for novel treatments for neurodegenerative diseases. Flavonoids, a group of compounds mainly derived from vegetables, share a resemblance, chemically, to estrogen, and indeed, some have been used as estrogen substitutes. To search for potential therapeutic agents against neurodegenerative diseases, different subclasses of flavonoids were analyzed and compared with estrogen. First, the estrogenic activities of these flavonoids were determined by activating the estrogen-responsive elements in cultured MCF-7 breast cancer cells. Second, the neuroprotective effects of flavonoids were revealed by measuring its inhibition effects on the formation of reactive oxygen species, the aggregation of beta-amyloid, and the induction of cell death by beta-amyloid in cultured neuronal PC12 cells. Among these flavonoids, baicalein, scutellarin, Hibifolin, and quercetin-3'-glucoside possessed the strongest effect in neuroprotection; however, the neuroprotective activity did not directly correlate with the estrogenic activity of the flavonoids. Identification of these flavonoids could be very useful in finding potential drugs, or food supplements, for treating Alzheimer's disease.
SPE-HPLC method for the determination of four flavonols in rat plasma and urine after oral administration of Abelmoschus manihot extract.[Pubmed:17258944]
J Chromatogr B Analyt Technol Biomed Life Sci. 2007 Jun 1;852(1-2):108-14.
A SPE-HPLC method was developed and validated for the simultaneous determination of flavonols, isoquercitrin (1), Hibifolin (2), myricetin (3), quercetin-3'-O-d-glucoside (4) and quercetin (5) in rat plasma and urine after oral administration of the total flavonoids from Abelmoschus manihot (TFA). The astragalin (6) and kaempferol (7) were used as internal standards (IS). Plasma and urine samples were pretreated by solid-phase extraction using Winchem C(18) reversed-phase cartridges. Analysis of the plasma and urinary extract was performed on YMC-Pack ODS-A C(18) and Thermo ODS-2HYEPRSIL C(18) reversed-phase column, respectively and a mobile phase of acetonitrile-0.1% phosphoric acid was employed. HPLC analysis was conducted with different elution gradients. The flow rate was 1.0 mL/min and the detection wavelength was set at 370 nm. Calibration ranges in plasma for flavonols 2-5 were at 0.011-2.220, 0.014-2.856, 0.022-4.320, and 0.028-5.600 microg/mL, respectively. In urine calibration ranges for flavonols 1, 2, 4 and 5 were at 2.00-16.00, 8.56-102.72, 2.70-21.60, and 3.00-24.00 microg/mL, respectively. The RSD of intra- and inter-day was less than 5.40% and 4.89% in plasma, and less than 3.96% and 6.85% in urine for all the analyses. A preliminary experiment to investigate the plasma concentration and urinary excretion of the flavonols after oral administration of TFA to rats demonstrated that the present method was suitable for determining the flavonols in rat plasma and urine.
Influence of a series of natural flavonoids on free radical generating systems and oxidative stress.[Pubmed:7975732]
Xenobiotica. 1994 Jul;24(7):689-99.
1. A series of flavonoids isolated from Indian medicinal plants: kaempferol-3-O-galactoside, hispidulin, nepetin, scutellarein, scutellarein-7-O-glucuronide, Hibifolin and morelloflavone were studied for their activity as inhibitors of microsomal lipid peroxidation and scavengers of oxygen free radicals in vitro as well as in a model of xenobiotic toxicity in mouse. 2. All compounds inhibited lipid peroxidation in vitro. The most potent compounds were nepetin (non-enzymic lipid peroxidation) and morelloflavone (enzymic lipid peroxidation) with IC50's in the micromolar range. Some of the compounds behaved as scavengers of hydroxyl radical in the deoxyribose degradation assay, with a calculated rate constant for kaempferol-3-O-galactoside of 1.55 x 10(10) M-1 s-1. 3. Scutellarein and nepetin were found to be inhibitors of xanthine oxidase activity, whereas morelloflavone acted as a scavenger of superoxide generated by hypoxanthine/xanthine oxidase. 4. Treatment of mice with scutellarein, hispidulin, nepetin and kaempferol-3-O-galactoside after bromobenzene intoxication decreased serum glumate-pyruvate transaminase activity, although only the last flavonoid was able to significantly reduce hepatic lipid peroxidation products and to increase the reduced glutathione level. In contrast, morelloflavone increased bromobenzene toxicity.
Effects of flavonoids on Naja naja and human recombinant synovial phospholipases A2 and inflammatory responses in mice.[Pubmed:8190018]
Life Sci. 1994;54(20):PL333-8.
Six flavonoid derivatives were tested for their influence on Naja naja and human recombinant synovial phospholipase A2. They showed a selectivity for the last enzyme with IC50 = 14.3, 17.6, 12.2 and 28.2 microM for quercetagetin, kaempferol-3-O-galactoside, scutellarein and scutellarein-7-O-glucuronide, respectively, while reduced effects were observed for hispidulin and Hibifolin. After topical application all the flavonoids inhibited 12-O-tetradecanoylphorbol-13-acetate-induced ear oedema in mice with a potency comparable to that of indomethacin and they were also able to inhibit carrageenan-induced mouse paw oedema at a dose of 150 mg/kg p.o. The blockade of the free hydroxyl at C-7 or C-6 reduced the anti-inflammatory activity and also the inhibitory effect on human recombinant synovial phospholipase A2. These results are in accordance with the notion that group II phospholipases A2 may play a role in experimental inflammation, although several mechanisms seems to be involved in the anti-inflammatory effect of this group of flavonoids.
Anti-inflammatory activity and inhibition of arachidonic acid metabolism by flavonoids.[Pubmed:1650522]
Agents Actions. 1991 Mar;32(3-4):283-8.
A group of flavonoids isolated from medicinal plants and which are selective inhibitors of lipoxygenase activity in vitro: sideritoflavone, cirsiliol, hypolaetin-8-O-beta-D-glucoside, hypolaetin, oroxindin, quercetagetin-7-O-beta-D-glucoside, gossypin, Hibifolin and gossypetin, besides leucocyanidol, have been studied for their effects on acute responses induced by carrageenin in mice. The oral administration of flavonoids to mice inhibited dose-dependently the development of paw oedema at 1, 3 and 5 h after carrageenin injection. A similar administration of flavonoids induced a dose-dependent inhibition of leukocyte accumulation in inflammatory exudates following intraperitoneal injection of carrageenin into mice. Some of the flavonoids exhibited a potency against leukocyte infiltration similar to that seen for inhibition of carrageenin oedema at 3 h of induction. In agreement with data reported in rats, indomethacin was much more effective on inhibition of prostaglandin E2 (PGE2) formation than on leukocyte infiltration in mice. The selectivity of flavonoids towards lipoxygenase is not retained in vivo since they behave as dual inhibitors of PGE2 and leukotriene B4 (LTB4) formation in peritoneal exudates. Our data support the inhibition of arachidonic acid metabolism as one of the mechanisms by which flavonoids exert their anti-inflammatory effects.


