CapsanthinCAS# 465-42-9 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 465-42-9 | SDF | Download SDF |
| PubChem ID | 5281228 | Appearance | Powder |
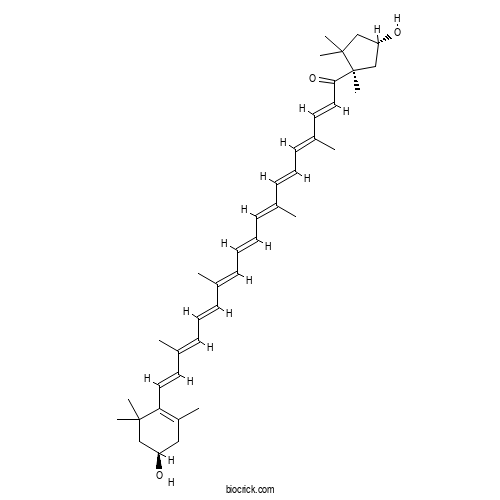
| Formula | C40H56O3 | M.Wt | 584.9 |
| Type of Compound | Miscellaneous | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | (2E,4E,6E,8E,10E,12E,14E,16E,18E)-19-[(4R)-4-hydroxy-2,6,6-trimethylcyclohexen-1-yl]-1-[(1R,4S)-4-hydroxy-1,2,2-trimethylcyclopentyl]-4,8,13,17-tetramethylnonadeca-2,4,6,8,10,12,14,16,18-nonaen-1-one | ||
| SMILES | CC1=C(C(CC(C1)O)(C)C)C=CC(=CC=CC(=CC=CC=C(C)C=CC=C(C)C=CC(=O)C2(CC(CC2(C)C)O)C)C)C | ||
| Standard InChIKey | VYIRVAXUEZSDNC-RDJLEWNRSA-N | ||
| Standard InChI | InChI=1S/C40H56O3/c1-29(17-13-19-31(3)21-23-36-33(5)25-34(41)26-38(36,6)7)15-11-12-16-30(2)18-14-20-32(4)22-24-37(43)40(10)28-35(42)27-39(40,8)9/h11-24,34-35,41-42H,25-28H2,1-10H3/b12-11+,17-13+,18-14+,23-21+,24-22+,29-15+,30-16+,31-19+,32-20+/t34-,35+,40+/m1/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Capsanthin has antioxidant activity. | |||||

Capsanthin Dilution Calculator

Capsanthin Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 1.7097 mL | 8.5485 mL | 17.0969 mL | 34.1939 mL | 42.7423 mL |
| 5 mM | 0.3419 mL | 1.7097 mL | 3.4194 mL | 6.8388 mL | 8.5485 mL |
| 10 mM | 0.171 mL | 0.8548 mL | 1.7097 mL | 3.4194 mL | 4.2742 mL |
| 50 mM | 0.0342 mL | 0.171 mL | 0.3419 mL | 0.6839 mL | 0.8548 mL |
| 100 mM | 0.0171 mL | 0.0855 mL | 0.171 mL | 0.3419 mL | 0.4274 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Tripalmitin
Catalog No.:BCN9736
CAS No.:555-44-2
- 1-(3',5'-dimethoxy)phenyl-2-[4''-O-beta-D-glucopyranosyl (6->1)-O-alpha-L-rhamnopyranosyl]phenylethane
Catalog No.:BCN9735
CAS No.:1338076-61-1
- gamma-Nonanolactone
Catalog No.:BCN9734
CAS No.:104-61-0
- Furfuryl alcohol
Catalog No.:BCN9733
CAS No.:98-00-0
- Carvacryl acetate
Catalog No.:BCN9732
CAS No.:6380-28-5
- Rauhimbine
Catalog No.:BCN9731
CAS No.:66634-44-4
- 11-Oxomogroside IIIE
Catalog No.:BCN9730
CAS No.:2096516-68-4
- 4-(2,6,6-Trimethyl-1-cyclohexenyl)-3-buten-2-one
Catalog No.:BCN9909
CAS No.:79-77-6
- Artanomaloide
Catalog No.:BCN9728
CAS No.:112823-41-3
- Phyllanthurinolactone
Catalog No.:BCN9727
CAS No.:168180-12-9
- Pumiloside
Catalog No.:BCN9726
CAS No.:126722-26-7
- Rossicaside B
Catalog No.:BCN9725
CAS No.:80458-55-5
- Uzarin
Catalog No.:BCN9738
CAS No.:20231-81-6
- Ethyl 3-hydroxybenzoate
Catalog No.:BCN9739
CAS No.:7781-98-8
- Clausine E
Catalog No.:BCN9740
CAS No.:182261-83-2
- Quercetin 3,4'-diglucoside
Catalog No.:BCN9741
CAS No.:29125-80-2
- 1-(2-Hydroxyphenyl)-3-phenyl-2-propenone
Catalog No.:BCN9742
CAS No.:1214-47-7
- Betonicine
Catalog No.:BCN9743
CAS No.:515-25-3
- (+)-atechin 5-gallate
Catalog No.:BCN9744
CAS No.:128232-62-2
- Hibifolin
Catalog No.:BCN9745
CAS No.:55366-56-8
- 2-Naphthol
Catalog No.:BCN9746
CAS No.:135-19-3
- 7-Methoxyflavonol
Catalog No.:BCN9747
CAS No.:7478-60-6
- Homoembelin
Catalog No.:BCN9748
CAS No.:38363-99-4
- Neosalvianen
Catalog No.:BCN9749
CAS No.:790673-00-6
Effects of paprika carotenoid supplementation on bone turnover in postmenopausal women: a randomized, double-blind, placebo-controlled, parallel-group comparison study.[Pubmed:33240029]
Food Nutr Res. 2020 Oct 6;64. pii: 4565.
Background: Paprika (Capsicum annuum L.) is a good source of carotenoids, including Capsanthin, beta-carotene, beta-cryptoxanthin, and zeaxanthin. Several epidemiological studies have shown a beneficial association of intake of these carotenoids or their blood concentration with bone mineral density (BMD) and fracture risk. However, little information is available regarding the effect of intake of these carotenoids on bone metabolism in postmenopausal women. Objective: The objective of the present study was to investigate the effects of paprika carotenoid extract (PCE) on bone turnover in healthy, postmenopausal women. Design: We conducted a randomized, double-blind, placebo-controlled, parallel-group comparison study. One hundred participants were randomly assigned to PCE or placebo groups. Each group was given a 20 mg PCE (equivalent to 1.4 mg of carotenoids) a day or a placebo for 24 weeks. We measured bone resorption markers (tartrate-resistant acid phosphatase 5b [TRACP-5b] and serum type I collagen cross-linked N-telopeptide [sNTX]) at 12 and 24 weeks and bone formation markers (bone alkaline phosphatase and osteocalcin) at 24 weeks. Results: The percentage decrease of TRACP-5b at 24 weeks was significantly higher for PCE than the placebo. There were no significant differences in sNTX or bone formation markers, although PCE decreased each marker compared with the placebo. Conclusion: Our findings suggest that PCE supplementation suppresses bone resorption and contributes to maintaining bone quality in postmenopausal women.
Capsanthin Stimulates the Mitochondrial Apoptosis-Mediated Cell Death, following DNA Damage in MCF-7 Cells.[Pubmed:32933334]
Nutr Cancer. 2020 Sep 16:1-9.
Carotenoids found in fruits and vegetables are compounds with significant biological activities. Epidemiological studies report that these compounds have significant anticancer effects, as well reducing the risk of cancer. In the present study, we aimed to determine the effects of Capsanthin, an important carotenoid of paprika, on expressions of proteins playing roles in the mitochondrial apoptosis pathway, in addition to its possible cytotoxic and genotoxic effects in MCF-7 cells. Furthermore, possible oxidant/anti-oxidant roles of Capsanthin on MCF-7 cells were investigated. The viability of MCF-7 cells was significantly decreased after 24 h of Capsanthin application. After Comet analysis, it was determined that the Capsanthin caused DNA damage on a dose-dependent manner. Furthermore, Western blot analysis showed that Capsanthin application increased p53 and Bax protein expressions and caused a decrease in Bcl-2 protein level. Capsanthin treatment decreased catalase and glutathione levels but increased lipid peroxidation. These results show that the Capsanthin causes oxidative stress and DNA damage, and increases mitochondrial apoptotic mechanism-mediated cell death after p53 and Bax protein activations.
Safety and efficacy of saponified paprika extract, containing capsanthin as main carotenoid source, for poultry for fattening and laying (except turkeys).[Pubmed:32874232]
EFSA J. 2020 Feb 24;18(2):e06023.
Following a request from the European Commission, the Panel on Additives and Products or Substances used in Animal Feed (FEEDAP) was asked to deliver a scientific opinion on the safety and efficacy of saponified paprika extract, containing Capsanthin as main carotenoid source, for poultry for fattening and laying (except turkeys). The saponified paprika (Capsicum annuum) extract contains various carotenoids at a concentration of 25-90 g/kg of which Capsanthin being the major one with quantity specified as > 35% of total carotenoids (TC). The maximum recommended use level of 40 mg TC/kg feed is safe for chickens for fattening and laying hens. The margin of safety is at least 6. This conclusion is extrapolated to minor poultry species for fattening and laying. The saponified paprika extract is not genotoxic. Based on the no observed effect level (NOEL) of the 90-day study in rat and the exposure estimates, the Panel considered that there would be an adequate margin of exposure (between 700 and 2000) to conclude that the level of exposure to residues of the saponified paprika (C. annuum) extract (Capsanthin not less than 35% of TCs) in animal tissues and products does not raise concern for the safety for the consumer. The saponified paprika extract is a viscous paste and as such users will not be exposed by inhalation. The applicant recognises that the extract may be irritant to skin and eyes. The FEEDAP Panel cannot conclude on the potential of any preparation to be toxic by inhalation, skin/eye irritant or skin sensitiser since no data were submitted. The use of saponified paprika extract in poultry feed raised no concern for the environment. Saponified paprika extract has the potential to pigment broiler skin and egg yolk. This conclusion is extrapolated to minor poultry species for fattening and laying.
Assessment of carotenoid concentrations in red peppers (Capsicum annuum) under domestic refrigeration for three weeks as determined by HPLC-DAD.[Pubmed:32514498]
Food Chem X. 2020 May 28;6:100092.
Red peppers (Capsicum annuum) are rich in carotenoids and are widely grown and consumed all over the world. Today's consumption patterns are characterized by periodical purchases of food and longer food storage periods, including raw fruits and vegetables, which could have a negative effect on healthy components. This study aims to investigate the individual carotenoid content in Lamuyo-variety red peppers in cool storage (7 degrees C) for three weeks. Carotenoid concentrations expressed in microg/100g of the edible portion were; lutein (1203), zeaxanthin (853), alpha-carotene (272), beta-carotene (2167), beta-cryptoxanthin (525), violaxanthin (770), Capsanthin (9667), phytoene (348) and phytofluene (143). Carotenoid concentrations did not significantly vary after 21 days under household refrigeration conditions and thus the nutritional supply of provitamin A carotenoids and of carotenoids with eye health benefits such as lutein and zeaxanthin, as well as others with potential health benefits in humans such as Capsanthin, violaxathin, phytoene and phytofluene.
Whole genome resequencing of four Italian sweet pepper landraces provides insights on sequence variation in genes of agronomic value.[Pubmed:32514106]
Sci Rep. 2020 Jun 8;10(1):9189.
Sweet pepper (Capsicum annuum L.) is a high value crop and one of the most widely grown vegetables belonging to the Solanaceae family. In addition to commercial varieties and F1 hybrids, a multitude of landraces are grown, whose genetic combination is the result of hundreds of years of random, environmental, and farmer selection. High genetic diversity exists in the landrace gene pool which however has scarcely been studied, thus bounding their cultivation. We re-sequenced four pepper inbred lines, within as many Italian landraces, which representative of as many fruit types: big sized blocky with sunken apex ('Quadrato') and protruding apex or heart shaped ('Cuneo'), elongated ('Corno') and smaller sized sub-spherical ('Tumaticot'). Each genomic sequence was obtained through Illumina platform at coverage ranging from 39 to 44x, and reconstructed at a chromosome scale. About 35.5k genes were predicted in each inbred line, of which 22,017 were shared among them and the reference genome (accession 'CM334'). Distinctive variations in miRNAs, resistance gene analogues (RGAs) and susceptibility genes (S-genes) were detected. A detailed survey of the SNP/Indels occurring in genes affecting fruit size, shape and quality identified the highest frequencies of variation in regulatory regions. Many structural variations were identified as presence/absence variations (PAVs), notably in resistance gene analogues (RGAs) and in the Capsanthin/capsorubin synthase (CCS) gene. The large allelic diversity observed in the four inbred lines suggests their potential use as a pre-breeding resource and represents a one-stop resource for C. annuum genomics and a key tool for dissecting the path from sequence variation to phenotype.
Candidate Gene Analysis Reveals That the Fruit Color Locus C1 Corresponds to PRR2 in Pepper (Capsicum frutescens).[Pubmed:32328078]
Front Plant Sci. 2020 Apr 9;11:399.
The diverse fruit colors of peppers (Capsicum spp.) are due to variations in carotenoid composition and content. Mature fruit color in peppers is regulated by three independent loci, C1, C2, and Y. C2 and Y encode phytoene synthase (PSY1) and Capsanthin-capsorubin synthase (CCS), respectively; however, the identity of the C1 gene has been unknown. With the aim of identifying C1, we analyzed two pepper accessions with different fruit colors: Capsicum frutescens AC08-045 and AC08-201, whose fruits are light yellow and white, respectively. Ultra-performance liquid chromatography showed that the total carotenoid content was six times higher in AC08-045 than in AC08-201 fruits, with similar composition of main carotenoids and slight difference in minor components. These results suggest that a genetic factor in AC08-201 may down-regulate overall carotenoid biosynthesis. Analyses of candidate genes related to carotenoid biosynthesis and plastid abundance revealed that both accessions carry non-functional alleles of CCS, golden2-like transcription factor (GLK2), and PSY1. However, a nonsense mutation (C2571T) in PRR2, a homolog of Arabidopsis pseudo response regulator2-like (APRR2), was present in only AC08-201. In a population derived from a cross between AC08-045 and AC08-201, a SNP marker based on the nonsense mutation co-segregated fully with fruit color, implying that the mutation in PRR2 may cause the white color of AC08-201 fruits. Transmission electron microscopy (TEM) of AC08-201 fruit pericarp also showed less developed granum structure in chloroplast and smaller plastoglobule in chromoplast compared to those of AC08-045. Virus-induced gene silencing (VIGS) of PRR2 significantly reduced carotenoid accumulation in Capsicum annuum 'Micropep Yellow', which carries non-functional mutations in both PSY1 and CCS. Furthermore, sequence analysis of PSY1, CCS, and PRR2 in other white pepper accessions of C. annuum and Capsicum chinense showed that they commonly have non-functional alleles in PSY1, CCS, and PRR2. Thus, our data demonstrate that the fruit color locus C1 in Capsicum spp. corresponds to the gene PRR2.
Phytoene synthase 2 can compensate for the absence of PSY1 in the control of color in Capsicum fruit.[Pubmed:32219321]
J Exp Bot. 2020 Jun 22;71(12):3417-3427.
Phytoene synthase 1 (PSY1) and Capsanthin-capsorubin synthase (CCS) are two major genes responsible for fruit color variation in pepper (Capsicum spp.). However, the role of PSY2 remains unknown. We used a systemic approach to examine the genetic factors responsible for the yellow fruit color of C. annuum 'MicroPep Yellow' (MY) and to determine the role of PSY2 in fruit color. We detected complete deletion of PSY1 and a retrotransposon insertion in CCS. Despite the loss of PSY1 and CCS function, both MY and mutant F2 plants from a cross between MY and the 'MicroPep Red' (MR) accumulated basal levels of carotenoids, indicating that other PSY genes may complement the loss of PSY1. qRT-PCR analysis indicated that PSY2 was constitutively expressed in both MR and MY fruits, and a color complementation assay using Escherichia coli revealed that PSY2 was capable of biosynthesizing a carotenoid. Virus-induced gene silencing of PSY2 in MY resulted in white fruits. These findings indicate that PSY2 can compensate for the absence of PSY1 in pepper fruit, resulting in the yellow color of MY fruits.
Influence of Postharvest Temperatures on Carotenoid Biosynthesis and Phytochemicals in Mature Green Chili (Capsicum annuum L.).[Pubmed:32121591]
Antioxidants (Basel). 2020 Mar 1;9(3). pii: antiox9030203.
An intense red color appearance in hot chili is what industry commonly demands. The harvested mature green "Takanotsume" chili, a popular cultivar in Japan, incubated at 20 and 30 degrees C is investigated. At 30 degrees C, the chili rapidly degraded chlorophylls and obtained an intense red color, but presented an orange-red color at 20 degrees C. The sample showed higher carotenoid accumulations at 30 degrees C, along with significantly upregulated carotenoid biosynthesis-related genes-phytoene synthase (Psy), lycopene-beta-cyclase (Lcyb), beta-carotene hydroxylase (CrtZ), and Capsanthin/capsorubin synthase (Ccs)-during the experiment. While the expression of the Ccs gene was reduced, there was a 5.5-fold upregulation of the Psy gene at the end of incubation. At 20 degrees C, the Psy gene was downregulated. These observations suggest that the expression of individual genes is temperature-dependent, and these would affect specific carotenoid compounds. The antioxidant capacity (2,2-diphenyl-1-picrylhydrazyl; DPPH and ferric-reducing antioxidant power; FRAP) values had no difference between temperatures; the higher content of total phenolics and vitamin C presented in the chili at 30 degrees C probably corresponds to the advanced ripening process. Thus, 30 degrees C is the recommended incubation temperature for mature green chili to achieve the industry-demanded intense red color and high accumulation of phytochemicals.
Genetic mapping of the c1 locus by GBS-based BSA-seq revealed Pseudo-Response Regulator 2 as a candidate gene controlling pepper fruit color.[Pubmed:32088729]
Theor Appl Genet. 2020 Jun;133(6):1897-1910.
KEY MESSAGE: The Pseudo-Response Regulator 2 gene was identified in the c1 locus, representing a genetic factor regulating fruit color in pepper using GBS-based BSA-seq. The loci c1, c2, and y have been widely reported as genetic determinants of various ripe fruit colors in pepper. However, c1, which may impact reduced pigmentation in red, orange, and yellow fruits, is not well understood. Two cultivars showing peach or orange fruit in Capsicum chinense 'Habanero' were found to have c2 mutation and were hypothesized to segregate c1 locus in the F2 population. Habanero peach (HP) showed a reduced level of chlorophylls, carotenoids and total soluble solids in immature and ripe fruits. A microscopic examination of the fruit pericarps revealed smaller plastids and less stacked thylakoid grana in HP. The expression of many genes related to chlorophyll and carotenoid biosynthetic pathways were reduced in HP. To identify the genomic region of the c1 locus, bulked segregant analysis combined with genotyping-by-sequencing was employed on an F2 population derived from a cross between Habanero orange and HP. One SNP at chromosome 1 was strongly associated with the peach fruit color. Pepper Pseudo-Response Regulator 2 (PRR2) was located close to the SNP and cosegregated with the peach fruit color. A 41 bp deletion at the third exon-intron junction region of CcPRR2 in HP resulted in a premature termination codon. A nonsense mutation of CaPRR2 was found in C. annuum 'IT158782' which had white ripe fruit coupled with null mutations of Capsanthin-capsorubin synthase (y) and phytoene synthase 1 (c2). These results will be useful for the genetic improvement in fruit color and nutritional quality in pepper.
Study on the Synthesis, Antioxidant Properties, and Self-Assembly of Carotenoid-Flavonoid Conjugates.[Pubmed:32024181]
Molecules. 2020 Feb 1;25(3). pii: molecules25030636.
Flavonoids and carotenoids possess beneficial physiological effects, such as high antioxidant capacity, anticarcinogenic, immunomodulatory, and anti-inflammatory properties, as well as protective effects against UV light. The covalent coupling of hydrophobic carotenoids with hydrophilic flavonoids, such as daidzein and chrysin, was achieved, resulting in new amphipathic structures. 7-Azidohexyl ethers of daidzein and chrysin were prepared in five steps, and their azide-alkyne [4 + 2] cycloaddition with pentynoates of 8'-apo-beta-carotenol, zeaxanthin, and Capsanthin afforded carotenoid-flavonoid conjugates. The trolox-equivalent antioxidant capacity against ABTS(*+) radical cation and self-assembly of the final products were examined. The 1:1 flavonoid-carotenoid hybrids generally showed higher antioxidant activity than their parent flavonoids but lower than that of the corresponding carotenoids. The diflavonoid hybrids of zeaxanthin and Capsanthin, however, were found to exhibit a synergistic enhancement in antioxidant capacities. ECD (electronic circular dichroism) and UV-vis analysis of zeaxanthin-flavonoid conjugates revealed that they form different optically active J-aggregates in acetone/water and tetrahydrofuran/water mixtures depending on the solvent ratio and type of the applied aprotic polar solvent, while the Capsanthin derivatives showed no self-assembly. The zeaxanthin bis-triazole conjugates with daidzein and with chrysin, differing only in the position of a phenolic hydroxyl group, showed significantly different aggregation profile upon the addition of water.
Capsanthin extract prevents obesity, reduces serum TMAO levels and modulates the gut microbiota composition in high-fat-diet induced obese C57BL/6J mice.[Pubmed:31955744]
Food Res Int. 2020 Feb;128:108774.
The present study investigated the anti-obesity effects and its mechanism of Capsanthin (CAP) in high-fat diet-induced obese C57BL/6J mice. Compared with untreated mice on a high-fat diet for 12weeks, CAP at 200mgkg(-1) reduced the body weight by 27.5%, significantly reversed glucose tolerance, effectively decreased the serum triglycerides, total cholesterol, low-density lipoprotein cholesterol, and trimethylamine N-oxide levels, markedly increased microbial diversity. Furthermore, 16S rRNA gene sequencing of the cecal microbiota suggested that CAP increased the abundance of Bacteroidetes, Bifidobacterium and Akkermansia, decreased the abundance of Ruminococcus and the ratio of Firmicutes/Bacteroidetes. Moreover, predicted functional domain analysis indicated that CAP increased the gene abundance of replication and repair, and decreased the gene abundance of membrane transports and carbohydrate metabolisms. Therefore, it seems CAP exhibit anti-obesity effect and might be used as a potential agent against obesity.
DNA methylation is involved in the regulation of pepper fruit ripening and interacts with phytohormones.[Pubmed:31907544]
J Exp Bot. 2020 Mar 25;71(6):1928-1942.
There is growing evidence to suggest that epigenetic tags, especially DNA methylation, are critical regulators of fruit ripening. To examine whether this is the case in sweet pepper (Capsicum annuum) we conducted experiments at the transcriptional, epigenetic, and physiological levels. McrBC PCR, bisulfite sequencing, and real-time PCR demonstrated that DNA hypomethylation occurred in the upstream region of the transcription start site of some genes related to pepper ripening at the turning stage, which may be attributed to up-regulation of CaDML2-like and down-regulation of CaMET1-like1, CaMET1-like2, CaCMT2-like, and CaCMT4-like. Silencing of CaMET1-like1 by virus-induced gene silencing led to DNA hypomethylation, increased content of soluble solids, and accumulation of carotenoids in the fruit, which was accompanied by changes in expression of genes involved in Capsanthin/capsorubin biosynthesis, cell wall degradation, and phytohormone metabolism and signaling. Endogenous ABA increased during fruit ripening, whereas endogenous IAA showed an opposite trend. No ethylene signal was detected during ripening. DNA hypomethylation repressed the expression of auxin and gibberellin biosynthesis genes as well as cytokinin degradation genes, but induced the expression of ABA biosynthesis genes. In mature-green pericarp, exogenous ABA induced expression of CaDML2-like but repressed that of CaCMT4-like. IAA treatment promoted the transcription of CaMET1-like1 and CaCMT3-like. Ethephon significantly up-regulated the expression of CaDML2-like. Treatment with GA3 and 6-BA showed indistinct effects on DNA methylation at the transcriptional level. On the basis of the results, a model is proposed that suggests a high likelihood of a role for DNA methylation in the regulation of ripening in the non-climacteric pepper fruit.
Rapid Determination of 17 Phthalate Esters in Capsanthin by QuEChERS Coupled with Gas Chromatography-Mass Spectrometry.[Pubmed:31904006]
Anal Sci. 2020 Apr 10;36(4):485-490.
A method for the simultaneous determination of 17 kinds of phthalate esters in Capsanthin was developed by the QuEChERS (quick, easy, cheap, effective, ruggedand safe) pretreatment method coupled with gas chromatography-mass spectrometry(GC-MS). Capsanthin samples were extracted with acetonitrile, and then sodium chloride and anhydrous magnesium sulfate were added for salting out. After the extracting liquids were cleansed by florisil, the supernatants were analyzed by GC-MS. The limits of detection (LOD) and limits of quantitation (LOQ) ranged from 0.2 to 0.5 mug/g and 0.6 to 1.5 mug/g, respectively. DMP, DEP, DAP, DIBP, DBP, BMPP, DPP, DHXP and DCHP were in the range of 0.2 - 10 mug/g; DMEP, DEEP, BBP, DBEP, DEHP, DPhP, DNOP and DNP were in the range of 0.5 - 20 mug/g. And all had good linearity and the linear correlation coefficients (R(2)) were more than 0.995. The average recoveries of 17 kinds of PAEs of the three levels were between 82.8 and 118.1%, and the relative standard deviations (RSDs) were between 0.12 and 7.3%. It is a simple, rapid, accurate and reliable method for the rapid detection of PAEs in large quantities of natural plant extract samples.
Color Development and Phytochemical Changes in Mature Green Chili (Capsicum annuum L.) Exposed to Red and Blue Light-Emitting Diodes.[Pubmed:31816240]
J Agric Food Chem. 2020 Jan 8;68(1):59-66.
Exposure of mature green "Takanotsume" chili fruit to blue and red light-emitting diodes (LEDs) was investigated. The red LED accelerated the red color development of chili as indicated by higher a* and chroma values, as well as lower hue angle and total chlorophyll compared to the blue LED and darkness (control). These were linked to increases in beta-carotene, free-Capsanthin, and total carotenoids. The carotenoid biosynthesis-related genes, lycopene-beta-cyclase (Lcyb), beta-carotene hydroxylase (CrtZ), and Capsanthin/capsolubin synthase (Ccs), were up-regulated by the red LED after 2 days of the experiment. The blue LED was more effective in increasing the expression of the phytoene synthase (Psy) gene at day 1 of experiment. The total phenolic, vitamin C content, and antioxidant capacity were also higher in the blue LED-treated chili. Results suggest that the responses of each carotenoid-related gene to the light wavelengths and the accumulation of phytochemicals are specific characteristics of this chili cultivar.
Xanthophyll: Health benefits and therapeutic insights.[Pubmed:31783054]
Life Sci. 2020 Jan 1;240:117104.
Xanthophylls constitute a major part of carotenoids in nature. They are an oxidized version of carotenoid. Xanthophyll has widely drawn scientists' attentions in terms of its functionality, bioavailability and diversity. An assortment of xanthophyll varieties includes lutein, zeaxanthin, beta-cryptoxanthin, Capsanthin, astaxanthin, and fucoxanthin. Chemically, lutein and zeaxanthin are dipolar carotenoids with hydroxyl groups at both ends of their molecules that bestow hydrophilic properties to them. Hydrophilic affinity in lutein and zeaxanthin makes better bioavailability in reaction with singlet oxygen in water phase, whereas non-polar carotenoids have shown to have less efficiency in scavenging free radicals. Xanthophylls have been studied for their effects in a wide variety of diseases including neurologic, ophthalmologic, oral, allergic and immune diseases. This review highlights pharmaco-pharmaceutical applications of xanthophylls as well asits drug interactions with beta-carotene. Different types of xanthophylls have been shown to have neuroprotective effects. Fucoxanthin demonstrated potent antiplasmodial activity. Lutein and zeaxanthin prevent the progression of age related macular degeneration. They have also demonstrated promising effects on uveitis, retinitis pigmentosa, scleritis, cataracts, glaucoma, retinal ischemia and choroideremia. Astaxanthin showed to have skin protecting effects against ultraviolet light injury. Astaxanthin have anti-allergic activity against the contact dermatitis especially to treat the patients having adverse reactions induced by steroids. Astaxanthin has been reported to exert beneficial effects in preventing oral lichen planus and early stage cancers. beta-cryptoxanthin has been considered a good candidate for prevention of bone loss via osteoblastic bone formation and inhibiting osteoclastic bone resorption. There is also some concern that higher dose of xanthophylls may be linked to increased risk of skin cancer and gastric adenocarcinoma. However this increased risk was not statistically significant when adjusted for confounding factors. Further researches including clinical studies are needed to better evaluate the efficacy and safety of xanthophylls in prevention and treatment of different diseases.


