UzarinCAS# 20231-81-6 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 20231-81-6 | SDF | Download SDF |
| PubChem ID | 20055063 | Appearance | White powder |
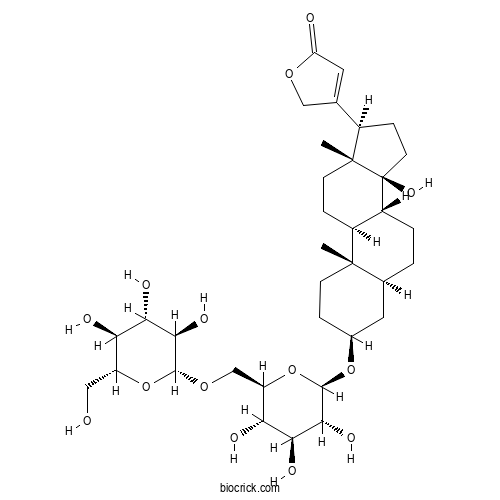
| Formula | C35H54O14 | M.Wt | 698.8 |
| Type of Compound | Steroids | Storage | Desiccate at -20°C |
| Synonyms | Uzarigenin 3-sophoroside | ||
| Solubility | Soluble in methanol and pyridine; practically insoluble in diethyl ether | ||
| Chemical Name | 3-[(3S,5S,8R,9S,10S,13R,14S,17R)-14-hydroxy-10,13-dimethyl-3-[(2R,3R,4S,5S,6R)-3,4,5-trihydroxy-6-[[(2R,3R,4S,5S,6R)-3,4,5-trihydroxy-6-(hydroxymethyl)oxan-2-yl]oxymethyl]oxan-2-yl]oxy-1,2,3,4,5,6,7,8,9,11,12,15,16,17-tetradecahydrocyclopenta[a]phenanthren-17-yl]-2H-furan-5-one | ||
| SMILES | CC12CCC(CC1CCC3C2CCC4(C3(CCC4C5=CC(=O)OC5)O)C)OC6C(C(C(C(O6)COC7C(C(C(C(O7)CO)O)O)O)O)O)O | ||
| Standard InChIKey | COIUWGNHAYDCDZ-RHWYPIQRSA-N | ||
| Standard InChI | InChI=1S/C35H54O14/c1-33-8-5-18(47-32-30(43)28(41)26(39)23(49-32)15-46-31-29(42)27(40)25(38)22(13-36)48-31)12-17(33)3-4-21-20(33)6-9-34(2)19(7-10-35(21,34)44)16-11-24(37)45-14-16/h11,17-23,25-32,36,38-44H,3-10,12-15H2,1-2H3/t17-,18-,19+,20-,21+,22+,23+,25+,26+,27-,28-,29+,30+,31+,32+,33-,34+,35-/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Reference standards. | |||||

Uzarin Dilution Calculator

Uzarin Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 1.431 mL | 7.1551 mL | 14.3102 mL | 28.6205 mL | 35.7756 mL |
| 5 mM | 0.2862 mL | 1.431 mL | 2.862 mL | 5.7241 mL | 7.1551 mL |
| 10 mM | 0.1431 mL | 0.7155 mL | 1.431 mL | 2.862 mL | 3.5776 mL |
| 50 mM | 0.0286 mL | 0.1431 mL | 0.2862 mL | 0.5724 mL | 0.7155 mL |
| 100 mM | 0.0143 mL | 0.0716 mL | 0.1431 mL | 0.2862 mL | 0.3578 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Capsanthin
Catalog No.:BCN9737
CAS No.:465-42-9
- Tripalmitin
Catalog No.:BCN9736
CAS No.:555-44-2
- 1-(3',5'-dimethoxy)phenyl-2-[4''-O-beta-D-glucopyranosyl (6->1)-O-alpha-L-rhamnopyranosyl]phenylethane
Catalog No.:BCN9735
CAS No.:1338076-61-1
- gamma-Nonanolactone
Catalog No.:BCN9734
CAS No.:104-61-0
- Furfuryl alcohol
Catalog No.:BCN9733
CAS No.:98-00-0
- Carvacryl acetate
Catalog No.:BCN9732
CAS No.:6380-28-5
- Rauhimbine
Catalog No.:BCN9731
CAS No.:66634-44-4
- 11-Oxomogroside IIIE
Catalog No.:BCN9730
CAS No.:2096516-68-4
- 4-(2,6,6-Trimethyl-1-cyclohexenyl)-3-buten-2-one
Catalog No.:BCN9909
CAS No.:79-77-6
- Artanomaloide
Catalog No.:BCN9728
CAS No.:112823-41-3
- Phyllanthurinolactone
Catalog No.:BCN9727
CAS No.:168180-12-9
- Pumiloside
Catalog No.:BCN9726
CAS No.:126722-26-7
- Ethyl 3-hydroxybenzoate
Catalog No.:BCN9739
CAS No.:7781-98-8
- Clausine E
Catalog No.:BCN9740
CAS No.:182261-83-2
- Quercetin 3,4'-diglucoside
Catalog No.:BCN9741
CAS No.:29125-80-2
- 1-(2-Hydroxyphenyl)-3-phenyl-2-propenone
Catalog No.:BCN9742
CAS No.:1214-47-7
- Betonicine
Catalog No.:BCN9743
CAS No.:515-25-3
- (+)-atechin 5-gallate
Catalog No.:BCN9744
CAS No.:128232-62-2
- Hibifolin
Catalog No.:BCN9745
CAS No.:55366-56-8
- 2-Naphthol
Catalog No.:BCN9746
CAS No.:135-19-3
- 7-Methoxyflavonol
Catalog No.:BCN9747
CAS No.:7478-60-6
- Homoembelin
Catalog No.:BCN9748
CAS No.:38363-99-4
- Neosalvianen
Catalog No.:BCN9749
CAS No.:790673-00-6
- Artemicapin C
Catalog No.:BCN9750
CAS No.:334007-19-1
Identification of potential SARS-CoV-2 inhibitors from South African medicinal plant extracts using molecular modelling approaches.[Pubmed:32839635]
S Afr J Bot. 2020 Sep;133:273-284.
The coronavirus is a group of viruses found in animals as well as humans and have been detected since the 1960s. However, a newly identified form, SARS-CoV-2, has triggered a recent pandemic of respiratory disease now called COVID-19. There is currently no specific antiviral drug for the treatment of this pandemic, with most treatment strategies focused on symptomatic management and supportive therapy. As such, several drug discovery efforts are ongoing for potent treatment agents, with medicinal plants gradually gaining prominence. Approximately 80% of the South African population use traditional medicines to meet their primary health care needs. The current study aimed to identify potential COVID-19 therapeutic agents from a list of 29 bioactive compounds isolated from commonly used South African medicinal plants using molecular docking and molecular dynamics. Molecular docking identified arabic acid from Acacia senegal and L-canavanine found in Sutherlandia frutescens as a potential inhibitor of SARS-CoV-2 3C-like main protease. Similarly, hypoxoside isolated from Hypoxis hemerocallidea and Uzarin from Xysmalobium undulatum, were identified as a potential inhibitor of SARS-CoV-2 receptor binding domain and SARS-CoV-2 RNA-dependent polymerase. These four bioactive compounds exhibited favourable binding orientations characterized by strong molecular interactions within respective inhibitors binding pockets of the target enzymes. Molecular dynamics simulations revealed that the binding of the identified inhibitors are characterized by structural perturbations which favour the inhibitory potency of these bioactive compounds. Additionally, in silico pharmacokinetic assessment of the compounds demonstrated favourable anti-SARS-CoV-2 properties. Although not conclusive, further experimental exploration of these compounds could serve as a starting point for the discovery of novel SARS-CoV-2 therapeutic.
Uzara - A quality control perspective of Xysmalobium undulatum.[Pubmed:26459659]
Pharm Biol. 2016 Jul;54(7):1272-9.
CONTEXT: Xysmalobium undulatum (L.) Aiton f var. (Asclepiadaceae), commonly known as uzara, is an ethnomedicinally important plant from southern Africa used to treat a variety of ailments. In addition to local use in African Traditional Medicine (ATM), formulations containing uzara have been successfully marketed by a number of pharmaceutical companies. Despite its commercialization, published adequate quality control (QC) protocols are lacking. OBJECTIVE: The study was conducted to develop QC protocols for uzara based on chromatographic and spectroscopic techniques. MATERIALS AND METHODS: High performance thin layer chromatography (HPTLC) and liquid chromatography coupled to mass spectrometry (LC-MS) were used to develop phytochemical fingerprints of ethanolic root extracts of 47 uzara samples collected from eight distinct localities in South Africa. Mid-infrared (MIR) spectroscopy was also explored as a suitable alternative technique for rapid and economic quantification of Uzarin. RESULTS: Adequate chromatographic profiles were obtained using both HPTLC and LC-MS analyses. The chromatographic patterns showed qualitative similarities among plants collected from different locations. The levels of Uzarin, the major constituent of uzara, were highly variable between locations, ranging from 17.8 to 139.9 mg/g (dry weight). A good coefficient of determination (R(2 )= 0.939) and low root mean square error of prediction (RMSEP = 7.9 mg/g) confirmed the accuracy of using MIR-PLS calibration models for the quantification of Uzarin. DISCUSSION AND CONCLUSION: Both HPTLC and LC-MS can be used as tools in developing quality control procedures for uzara. MIR in combination with chemometrics provides a fast alternative method for the quantification of Uzarin.
Analysis of proliferation and apoptotic induction by 20 steroid glycosides in 143B osteosarcoma cells in vitro.[Pubmed:26300346]
Cell Prolif. 2015 Oct;48(5):600-10.
OBJECTIVES: Osteosarcoma is the most common type of malignant bone tumour in children and adolescents; it has poor prognosis, is highly metastatic and is resistant to current therapeutic approaches. In this study, different herbal extracts used in phytotherapy have been screened after searching innovative natural anti-cancer components. MATERIALS AND METHODS: Twenty steroid glycosides were examined for accordance to their potential of inhibiting cell proliferation and inducing apoptosis in the osteosarcoma cell line 143B. Cell proliferation was examined using a CASY counter. Effects of cardiac glycosides on induction of apoptosis were evaluated by Annexin V-APC and flow cytometry, caspase activity assay and measurement of mitochondrial membrane potential. RESULTS: The study revealed that various steroid glycosides suppress cell proliferation in a concentration-dependent manner. Further investigations indicated apoptotic induction by 17 of the 20 tested cardenolides and bufadienolides. Bufadienolide proscillaridin A, arenobufagin, and cardenolides evomonoside, convallatoxol and ouabain waged strongest apoptotic induction, associated with breakdown of mitochondrial membrane potential and activation of caspases -8 and -9. In contrast, the bufadienolide resibufogenin and cardenolide Uzarin had no effect on proliferation inhibition, apoptotic induction or change in mitochondrial membrane potential. CONCLUSION: These results indicate that bufadienolides proscillaridin A and arenobufagin and cardenolide evomonoside, or related natural compounds might be promising new starting points for development of novel anti-cancer agents for treatment of osteosarcoma.
Xysmalobium undulatum (uzara) - review of an antidiarrhoeal traditional medicine.[Pubmed:25193007]
J Ethnopharmacol. 2014 Oct 28;156:135-46.
ETHNOPHARMACOLOGICAL RELEVANCE: Xysmalobium undulatum, commonly known as uzara, is traditionally used as an antidiarrhoeal and to treat stomach cramps, dysmenorrhoea and afterbirth cramps. In addition, it was reportedly used to treat anxiety and other conditions relating to mental health. AIM OF THE REVIEW: To unite the botanical aspects, ethnopharmacology, phytochemistry, biological activity, pharmacokinetic and pharmacodynamic data, toxicity and commercial aspects of the scientific literature available on uzara. METHOD: An extensive review of the literature covering 1917-2014 was carried out. Electronic databases including Scopus, Pubmed, Google Scholar and Google were used to assemble the data. All abstracts, full-text articles and books written in English and German were examined and included. RESULTS: The phytochemistry of uzara has been comprehensively investigated and at least 18 compounds have been isolated and characterised. Uzara contains mainly cardenolide glycosides such as Uzarin and xysmalorin and cardenolide aglycones such as uzarigenin and xysmalogenin. Limited scientific studies on the biological activity of uzara have been done. In vitro antisecretory antidiarrhoeal action was confirmed. Central nervous system activity was conflicting, in vitro and in vivo (animals) studies were inconclusive and no clinical studies have been performed. No antimutagenic effects have been reported and no toxicity up to date has been associated with uzara consumption. Significant cross-reactivity of uzara compounds with commercial digoxin and digitoxin assays may interfere with therapeutic drug monitoring. CONCLUSIONS: The key traditional uses associated with uzara have been investigated in vitro and in vivo (animal), but clinical trial data is lacking.
Cardiovascular effects, pharmacokinetics and cross-reactivity in digitalis glycoside immunoassays of an antidiarrheal uzara root extract.[Pubmed:22784612]
Int J Clin Pharmacol Ther. 2012 Oct;50(10):729-40.
Uzara glycosides (UG) extracted from Xysmalobium undulatum are used for treating non-specific diarrhea.Cross-reactivity has been described for UG in digitalis glycoside assays but digitalis-like cardiac effects are controversially discussed. Therefore, we performed a randomized, singleblind cross-over study in 18 healthy volunteers receiving a commercially available Uzara product (Uzara(R) Losung N, Stada AG, Bad Vilbel, Germany (ULN)), digoxin (1 mg, i.v., positive control) and placebo in double-dummy technique. Pharmacodynamic effects were quantified by means of ECG and impedance cardiography (ICG). After oral administration of ULN, main metabolites were determined using HPLC-MS/MS and digitalis-like serum levels (DLSL) were measured in two digitoxin and digoxin assays, respectively. In comparison to placebo, ULN did not change significantly any PD parameters whereas digoxin altered significantly area under the effect curve of several ECG and ICG parameters, respectively. Since some serum levels of three ULN ingredients (Uzarin, uzarigenin and xysmalorin) were below LLQ, PK analyses could only be performed for allouzarigenin and revealed a marked inter-individual variability. Therefore, median values (min; max) were calculated as follows: Cmax = 0.39 (0.15; 1.81) ng/ml, tmax = 7.0 (3.0; 36.0) h, T1/2 = 5.2 (0.8; 23.6) h, AUC0-36h = 4.2 (0.8; 11.1) ng/mlxh, AUC0-infinity = 5.8 (1.8; 13.1) ng/mlxh. DLSL reached Cmax of 28 ng/ml and 1,980 ng/ml for digoxin and digitoxin, respectively. We could not observe significant cardiovascular pharmacodynamic effects after oral administration of the recommended single dose of Uzara extract to healthy volunteers. However, considerable DLSL could be detected, proving cross-reactivity of uzara components with the conventional digitalis assays used. However, none of the metabolites we had suspected to be the cause for the crossreactivity could be identified in reasonable quantities.
Inhibition of Na+,K+-ATPase by the cardenolide 6'-O-(E-4-hydroxycinnamoyl) desglucouzarin.[Pubmed:9790942]
Biochem Biophys Res Commun. 1998 Oct 9;251(1):256-9.
Among the major cardenolides from the milkweed Asclepias asperula, 6'-O-(E-4-hydroxycinnamoyl) desglucoUzarin has not been characterized biochemically. In this study, its binding affinity for a physiological receptor, porcine kidney Na+,K+-ATPase, was found to be lower than the other cardenolides in this plant. The order of affinities from highest to lowest was: uzarigenin (Kd = 1.05 microM) = desglucoUzarin (Kd = 0.98 microM) > Uzarin (Kd = 4.0 microM) > 6'-O-(E-4-hydroxycinnamoyl) desglucoUzarin (Kd = 16 microM). The chemical attachment of the 4-hydroxycinnamoyl group to the 6'-carbon of desglucoUzarin significantly inhibits binding. This agrees with predictions that a 5'-methyl group on cardenolides fits the receptor site optimally for the porcine kidney enzyme. The 4-hydroxycinnamic ester was also found to be fluorescent.
Phytochemical reinvestigation of Xysmalobium undulatum roots (Uzara).[Pubmed:17252392]
Planta Med. 1997 Aug;63(4):343-6.
Four major cardenolide glycosides of Xysmalobium undulatum (L.) R. Br. roots (Uzara) have been isolated for the first time in pure form. The structures were elucidated by spectral analyses and determined as sophorosides and their 17-epimers, respectively. Thus, the structural elucidation of Uzarin and xysmalorin, the two main glycosides, has been completed. The corresponding H-17beta isomers, alloUzarin and alloxysmalorin, were characterized as genuine compounds.
Cardenolide fingerprint of monarch butterflies reared on common milkweed,Asclepias syriaca L.[Pubmed:24271887]
J Chem Ecol. 1989 Mar;15(3):819-53.
Monarch butterfly,Danaus plexippus (L.), larvae were collected during August 1983 from the common milkweed,Asclepias syriaca L., across its extensive North American range from North Dakota, east to Vermont, and south to Virginia. This confirms that the late summer distribution of breeding monarchs in eastern North America coincides with the range of this extremely abundant milkweed resource. Plant cardenolide concentrations, assayed by spectrophotometry in 158 samples from 27 collection sites, were biased towards plants with low cardenolide, and ranged from 4 to 229 mug/ 0.1 g dry weight, with a mean of 50 mug/0.1 g. Monarch larvae reared on these plants stored cardenolides logarithmically, and produced 158 adults with a normally distributed concentration range from 0 to 792 mug/0. l g dry butterfly, with a mean of 234 mug/0.1 g. Thus butterflies increased the mean plant cardenolide concentration by 4.7. The eastern plants and their resultant butterflies had higher cardenolide concentrations than those from the west, and in some areas monarchs sequestered more cardenolide from equivalent plants. Plants growing in small patches had higher cardenolide concentrations than those in larger patches, but this did not influence butterfly concentration. However, younger plants and those at habitat edges had higher cardenolide concentrations than either older, shaded, or open habitat plants, and this did influence butterfly storage. There were no apparent topographical differences reflected in the cardenolides of plants and butterflies. Twenty-eight cardenolides were recognized by thin-layer chromatography, with 27 in plants and 21 in butterflies. Butterflies stored cardenolides within the more polar 46% of the plantR d range, these being sequestered in higher relative concentrations than they occurred in the plants. By comparison with published TLC cardenolide mobilities, spots 3, 4, 9, 16, 24 or 25, 26, and 27, may be the cardenolides syrioside, Uzarin, syriobioside, syriogenin, uzarigenin, labriformidin, and labriformin, respectively. Cochromatography with cardenolide standards indicated that desglucosyrioside did not occur in the plants but did occur in 70% of the butterflies, and aspecioside was in 99% of the plants and 100% of the butterflies. The polar aspecioside was the single most concentrated and diagnostic cardenolide in both plants and butterflies. ButterflyR d values were dependent on those of the plant, and both showed remarkable uniformity over the range of areas sampled. Thus contrary to previous reports,A. syriaca has a biogeographically consistent cardenolide fingerprint pattern. The ecological implications of this for understanding the monarch's annual migration cycle are significant.


