7-MethoxyflavonolCAS# 7478-60-6 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 7478-60-6 | SDF | Download SDF |
| PubChem ID | 344546 | Appearance | Powder |
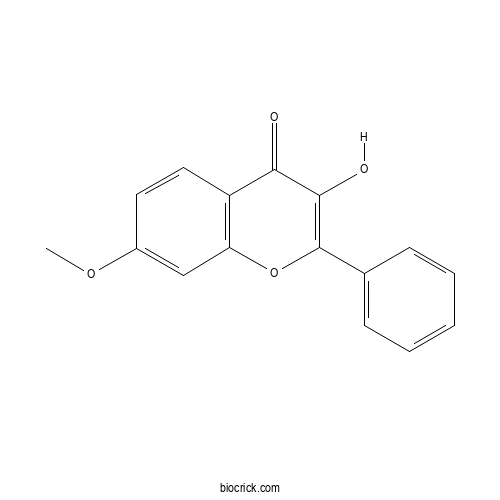
| Formula | C16H12O4 | M.Wt | 268.3 |
| Type of Compound | Flavonoids | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | 3-hydroxy-7-methoxy-2-phenylchromen-4-one | ||
| SMILES | COC1=CC2=C(C=C1)C(=O)C(=C(O2)C3=CC=CC=C3)O | ||
| Standard InChIKey | IPRIGHIBTRMTDP-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C16H12O4/c1-19-11-7-8-12-13(9-11)20-16(15(18)14(12)17)10-5-3-2-4-6-10/h2-9,18H,1H3 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Reference standards. | |||||

7-Methoxyflavonol Dilution Calculator

7-Methoxyflavonol Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.7272 mL | 18.6359 mL | 37.2717 mL | 74.5434 mL | 93.1793 mL |
| 5 mM | 0.7454 mL | 3.7272 mL | 7.4543 mL | 14.9087 mL | 18.6359 mL |
| 10 mM | 0.3727 mL | 1.8636 mL | 3.7272 mL | 7.4543 mL | 9.3179 mL |
| 50 mM | 0.0745 mL | 0.3727 mL | 0.7454 mL | 1.4909 mL | 1.8636 mL |
| 100 mM | 0.0373 mL | 0.1864 mL | 0.3727 mL | 0.7454 mL | 0.9318 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- 2-Naphthol
Catalog No.:BCN9746
CAS No.:135-19-3
- Hibifolin
Catalog No.:BCN9745
CAS No.:55366-56-8
- (+)-atechin 5-gallate
Catalog No.:BCN9744
CAS No.:128232-62-2
- Betonicine
Catalog No.:BCN9743
CAS No.:515-25-3
- 1-(2-Hydroxyphenyl)-3-phenyl-2-propenone
Catalog No.:BCN9742
CAS No.:1214-47-7
- Quercetin 3,4'-diglucoside
Catalog No.:BCN9741
CAS No.:29125-80-2
- Clausine E
Catalog No.:BCN9740
CAS No.:182261-83-2
- Ethyl 3-hydroxybenzoate
Catalog No.:BCN9739
CAS No.:7781-98-8
- Uzarin
Catalog No.:BCN9738
CAS No.:20231-81-6
- Capsanthin
Catalog No.:BCN9737
CAS No.:465-42-9
- Tripalmitin
Catalog No.:BCN9736
CAS No.:555-44-2
- 1-(3',5'-dimethoxy)phenyl-2-[4''-O-beta-D-glucopyranosyl (6->1)-O-alpha-L-rhamnopyranosyl]phenylethane
Catalog No.:BCN9735
CAS No.:1338076-61-1
- Homoembelin
Catalog No.:BCN9748
CAS No.:38363-99-4
- Neosalvianen
Catalog No.:BCN9749
CAS No.:790673-00-6
- Artemicapin C
Catalog No.:BCN9750
CAS No.:334007-19-1
- 3',4',5,5',6,7-Hexamethoxyflavone
Catalog No.:BCN9751
CAS No.:29043-07-0
- Benzyl acetate
Catalog No.:BCN9752
CAS No.:140-11-4
- (+)-Menthofuran
Catalog No.:BCN9753
CAS No.:17957-94-7
- Arganine B
Catalog No.:BCN9754
CAS No.:144425-21-8
- 5-Methoxyflavanone
Catalog No.:BCN9755
CAS No.:55947-36-9
- 6,7-Dihydroxyflavone
Catalog No.:BCN9756
CAS No.:38183-04-9
- Octanoic acid
Catalog No.:BCN9757
CAS No.:124-07-2
- Ruberythric acid
Catalog No.:BCN9758
CAS No.:152-84-1
- Allylcysteine
Catalog No.:BCN9759
CAS No.:21593-77-1
Antioxidant Flavonols and Phenolic Compounds from Atraphaxis frutescens and Their Inhibitory Activities against Insect Phenoloxidase and Mushroom Tyrosinase.[Pubmed:28006914]
J Nat Prod. 2016 Dec 23;79(12):3065-3071.
Chemical investigation of the aerial parts of Atraphaxis frutescens resulted in the isolation of five 7-Methoxyflavonols with pyrogallol B-ring moieties (1-5), a fisetinidol glucoside (13), and a benzyl glycoside (18), together with 26 known compounds including flavonoids, phenylpropanoid amides, anthraquinone glycosides, lignans, and a benzyl derivative. The principal chemical structural feature of the isolated compounds was either a pyrogallol or catechol B-ring moiety, and they showed potent 1,1-diphenyl-2-picrylhydrazyl radical scavenging activities. To assess the effects of these antioxidants on biological enzymes, their inhibitory effects against an insect phenoloxidase and a mushroom tyrosinase were evaluated. This study indicated that insect phenoloxidase was inhibited by phenylpropanoid amides and that mushroom tyrosinase was inhibited by the characteristic 7-Methoxyflavonol 3-O-rhamnopyranosides.
Isolation and characterization of structurally novel antimutagenic flavonoids from spinach (Spinacia oleracea).[Pubmed:11409964]
J Agric Food Chem. 2001 Jun;49(6):2767-73.
Thirteen compounds, isolated from spinach (Spinacia oleracea), acted as antimutagens against the dietary carcinogen 2-amino-3-methylimidazo[4,5-f]quinoline in Salmonella typhimurium TA 98. The antimutagens were purified by preparative and micropreparative HPLC from a methanol/water (70:30, v/v) extract of dry spinach (commercial product) after removal of lipophilic compounds such as chlorophylls and carotenoids by solid-phase extraction (SPE). Pure active compounds were identified by instrumental analysis including FT-IR, (1)H and (13)C NMR, UV-vis spectroscopy, and mass spectrometry. All of these compounds were flavonoids and related compounds that could be attributed to five groups: (A, methylenedioxyflavonol glucuronides) 5,3'-dihydroxy-4'-methoxy-6,7-methylenedioxyflavonol 3-O-beta-glucuronide (compound 1), 5,2',3'-trihydroxy-4'-methoxy-6,7-methylenedioxyflavonol 3-O-beta-glucuronide (compound 2), 5-hydroxy-3',4'-dimethoxy-6,7-methylenedioxyflavonol 3-O-beta-glucuronide (compound 3); (B, flavonol glucuronides) 5,6,3'-trihydroxy-7,4'-dimethoxyflavonol 3-O-beta-glucuronide (compound 4), 5,6-dihydroxy-7,3',4'-trimethoxyflavonol 3-O-beta-glucuronide (compound 5); (C, flavonol disaccharides) 5,6,4'-trihydroxy-7,3'-dimethoxyflavonol 3-O-disaccharide (compound 6), 5,6,3',4'-tetrahydroxy-7-Methoxyflavonol 3-O-disaccharide (compounds 7 and 8); (D, flavanones) 5,8,4'-trihydroxyflavanone (compound 9), 7,8,4'-trihydroxyflavanone (compound 10); (E, flavonoid-related compounds) compounds 11, 12, and 13 with incompletely elucidated structures. The yield of compound 1 was 0.3%, related to dry weight, whereas the yields of compounds 2-13 ranged between 0.017 and 0.069%. IC(50) values (antimutagenic potencies) of the flavonol glucuronides ranged between 24.2 and 58.2 microM, whereas the flavonol disaccharides (compounds 7 and 8), the flavanones (compounds 9 and 10), and the flavonoid-related glycosidic compounds 11-13 were only weakly active. The aglycons of compounds 7 and 8, however, were potent antimutagens (IC(50) = 10.4 and 13.0 microM, respectively).
Evaluation of the antioxidant potential of natural products.[Pubmed:10499128]
Comb Chem High Throughput Screen. 1998 Apr;1(1):35-46.
Since reactive oxygen radicals play an important role in carcinogenesis and other human disease states, antioxidants present in consumable fruits, vegetables, and beverages have received considerable attention as cancer chemopreventive agents. Thus, in order to identify antioxidants in plant extracts, test materials were assessed for potential to scavenge stable 1,2-diphenyl-2-picrylhydrazyl (DPPH) free radicals, reduce TPA-induced free radical formation in cultured HL-60 human leukemia cells, and inhibit responses observed with a xanthine/xanthine oxidase assay system. Approximately 700 plant extracts were evaluated, and 28 were found to be active in the DPPH free radical scavenging assay. Based on secondary analyses performed to assess inhibition of 7,12-dimethylbenz(a)anthracene-induced preneoplastic lesion formation with a mouse mammary organ culture model, Chorizanthe diffusa Benth. (Polygonaceae), Mezoneuron cucullatum Roxb. (Leguminosae), Cerbera manghas L. (Apocynaceae) and Daphniphyllum calycinum Benth. (Daphniphyllaceae) were selected and subjected to bioassay-guided fractionation. 5,7,3',5'-Tetrahydroxy-8,4'-dimethoxyflavonol, 5,8,4'-trihydroxy-7,3'-dimethoxyflavonol, 5,3',4'-trihydroxy-7-Methoxyflavonol, and 6,3',4'-trihydroxy-7-Methoxyflavonol were identified as active principles from C. diffusa. Piceatannol, trans-resveratrol, apigenin and scirpusin A were found as the active principles of M. cucullatum, olivil, (-)-carinol, and (+)-cycloolivil were active principles from C. manghas, and 5,6,7,4'-tetrahydroxyflavone 3-O-rutinoside and kaempferol 3-O-neohesperidoside were active principles from D. calycinum. Of these substances, the hydroxystilbenes piceatannol and transresveratrol have thus far been shown to inhibit carcinogen-induced preneoplastic lesion formation in the mouse mammary gland organ culture model.


