(+)-Bornyl acetateCAS# 20347-65-3 |
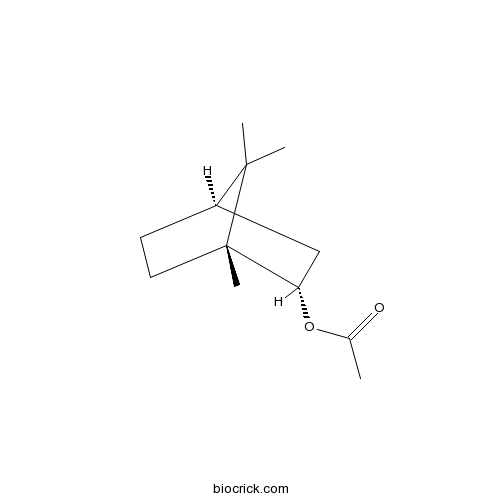
- Isobornyl acetate
Catalog No.:BCN8296
CAS No.:125-12-2
- (-)-Bornyl acetate
Catalog No.:BCN2636
CAS No.:5655-61-8
- Bornyl Acetate
Catalog No.:BCN9108
CAS No.:76-49-3
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 20347-65-3 | SDF | Download SDF |
| PubChem ID | 6950274 | Appearance | Liquid |
| Formula | C12H20O2 | M.Wt | 196.29 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | [(1R,2S,4R)-1,7,7-trimethyl-2-bicyclo[2.2.1]heptanyl] acetate | ||
| SMILES | CC(=O)OC1CC2CCC1(C2(C)C)C | ||
| Standard InChIKey | KGEKLUUHTZCSIP-SCVCMEIPSA-N | ||
| Standard InChI | InChI=1S/C12H20O2/c1-8(13)14-10-7-9-5-6-12(10,4)11(9,2)3/h9-10H,5-7H2,1-4H3/t9-,10+,12+/m1/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

(+)-Bornyl acetate Dilution Calculator

(+)-Bornyl acetate Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 5.0945 mL | 25.4725 mL | 50.945 mL | 101.8901 mL | 127.3626 mL |
| 5 mM | 1.0189 mL | 5.0945 mL | 10.189 mL | 20.378 mL | 25.4725 mL |
| 10 mM | 0.5095 mL | 2.5473 mL | 5.0945 mL | 10.189 mL | 12.7363 mL |
| 50 mM | 0.1019 mL | 0.5095 mL | 1.0189 mL | 2.0378 mL | 2.5473 mL |
| 100 mM | 0.0509 mL | 0.2547 mL | 0.5095 mL | 1.0189 mL | 1.2736 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- SNX 482
Catalog No.:BCC5952
CAS No.:203460-30-4
- 18-Norabieta-8,11,13-triene-4,15-diol
Catalog No.:BCN1504
CAS No.:203455-81-6
- Luteollin 5-glucoside
Catalog No.:BCN5391
CAS No.:20344-46-1
- 7-Oxo-beta-sitosterol
Catalog No.:BCN4891
CAS No.:2034-74-4
- Daphnoretin
Catalog No.:BCN2473
CAS No.:2034-69-7
- H-D-Arg-NH2.2HCl
Catalog No.:BCC2870
CAS No.:203308-91-2
- 3,4-Dimethoxyphenol
Catalog No.:BCN4890
CAS No.:2033-89-8
- 3,4,5-Trimethoxy-trans-cinnamic acid
Catalog No.:BCN3423
CAS No.:20329-98-0
- 3,5-Diacetamido-4-methylbenzoic acid
Catalog No.:BCN1505
CAS No.:6633-37-0
- Solamarine
Catalog No.:BCN3806
CAS No.:20318-30-3
- Tiliroside
Catalog No.:BCN4889
CAS No.:20316-62-5
- Procyanidin B1
Catalog No.:BCN6314
CAS No.:20315-25-7
- (-)-Maackiain
Catalog No.:BCN4892
CAS No.:2035-15-6
- Brefeldin A
Catalog No.:BCC4387
CAS No.:20350-15-6
- 2,4,6,6-Tetramethyl-3(6H)-pyridinone
Catalog No.:BCN4893
CAS No.:203524-64-5
- DMNB
Catalog No.:BCC7259
CAS No.:20357-25-9
- Hastacine
Catalog No.:BCN2086
CAS No.:20361-77-7
- Arctiin
Catalog No.:BCN1090
CAS No.:20362-31-6
- Istaroxime
Catalog No.:BCC1660
CAS No.:203737-93-3
- 27-Hydroxycholesterol
Catalog No.:BCN2750
CAS No.:20380-11-4
- MMAD
Catalog No.:BCC1774
CAS No.:203849-91-6
- Fmoc-ß-HoGlu(OtBu)-OH
Catalog No.:BCC3234
CAS No.:203854-49-3
- Helioxanthin derivative 5-4-2
Catalog No.:BCC5414
CAS No.:203935-39-1
- SU5416
Catalog No.:BCC1974
CAS No.:204005-46-9
Organoleptic Evaluation of Amomi Fructus and Its Further Background Verified via Morphological Measurement and GC Coupled with E-Nose.[Pubmed:29692854]
Evid Based Complement Alternat Med. 2018 Mar 5;2018:4689767.
The present study investigated the maneuverability and reasonability of sensory analysis, which has been applied in TCM identification for a long time. Ten assessors were trained and generated the human panel to carry out the organoleptic evaluation of twenty-five batches of Sha-Ren samples. Accordingly, samples were scored from 0 (lowest) to 10 (highest) for sensory attributes. Based on this, samples were divided into three classes: high class (Yang-Chun-Sha from Guang-Dong), moderate class (Yang-Chun-Sha samples from Yun-Nan and Guang-Xi), and low class (Lv-Qiao-Sha from marketplaces). For further background, three instrumental approaches were employed: morphological measurement with three indices (longitudinal diameter, transverse diameter, and 100-fruit weight), GC for determination of bornyl acetate contents, and E-nose for aromatic fingerprint. It is demonstrated in the results that GC and E-nose analyses were in great agreement with organoleptic evaluation. It gives insights into further studies on searching better morphological indicators and improving discriminant model of E-nose.
Bornyl acetate suppresses ox-LDL-induced attachment of THP-1 monocytes to endothelial cells.[Pubmed:29655164]
Biomed Pharmacother. 2018 Jul;103:234-239.
Leukocyte recruitment to the surface of the endothelium plays a pivotal role in the development of cardiovascular diseases. Bornyl acetate is the main volatile constituent present in numerous conifer oils, which has displayed its anti-oxidant and anti-inflammatory properties in different types of tissues and cells. However, little information regarding the effects of bornyl acetate on vascular endothelial inflammation has been reported before. In the current study, we aimed to investigate the pharmacological roles of bornyl acetate against ox-LDL-induced leukocyte adhesion to the endothelium. Our findings indicate that bornyl acetate ameliorated ox-LDL-induced reduction in cell viability of HUVECs. Additionally, bornyl acetate inhibited the attachment of THP-1 monocytes to HUVECs induced by treatment with ox-LDL through ameliorating the expression of ICAM-1, VCAM-1, and E-selectin. Mechanistically, we found that bornyl acetate could suppress activation of the IkappaBalpha/NF-kappaB signaling pathway. Lastly, our results indicate that bornyl acetate mitigated expression of the pro-inflammatory cytokines TNF-alpha and IL-1beta. Our results suggest the therapeutic potential of bornyl acetate in patients with atherosclerosis.
Effects of pure plant secondary metabolites on methane production, rumen fermentation and rumen bacteria populations in vitro.[Pubmed:29707819]
J Anim Physiol Anim Nutr (Berl). 2018 Aug;102(4):869-881.
In this study, the effects of seven pure plant secondary metabolites (PSMs) on rumen fermentation, methane (CH4 ) production and rumen bacterial community composition were determined. Two in vitro trials were conducted. In trial 1, nine concentrations of 8-hydroxyquinoline, alpha-terpineol, camphor, bornyl acetate, alpha-pinene, thymoquinone and thymol were incubated on separate days using in vitro 24-hr batch incubations. All compounds tested demonstrated the ability to alter rumen fermentation parameters and decrease CH4 production. However, effective concentrations differed among individual PSMs. The lowest concentrations that reduced (p < .05) CH4 production were as follows: 8 mg/L of 8-hydroxyquinoline, 120 mg/L of thymoquinone, 240 mg/L of thymol and 480 mg/L of alpha-terpineol, camphor, bornyl acetate and alpha-pinene. These concentrations were selected for use in trial 2. In trial 2, PSMs were incubated in one run. Methane was decreased (p < .05) by all PSMs at selected concentrations. However, only 8-hydroxyquinoline, bornyl acetate and thymoquinone decreased (p < .05) CH4 relative to volatile fatty acids (VFAs). Based on denaturing gradient gel electrophoresis analysis, different PSMs changed the composition of bacterial communities to different extents. As revealed by Ion Torrent sequencing, the effects of PSMs on relative abundance were most pronounced in the predominant families, especially in Lachnospiraceae, Succinivibrionaceae, Prevotellaceae, unclassified Clostridiales and Ruminococcaceae. The CH4 production was correlated negatively (-.72; p < .05) with relative abundance of Succinivibrionaceae and positively with relative abundance of Ruminococcaceae (.86; p < .05). In summary, this study identified three pure PSMs (8hydroxyquinoline, bornyl acetate and thymoquinone) with potentially promising effects on rumen CH4 production. The PSMs tested in this study demonstrated considerable impact on rumen bacterial communities even at the lowest concentrations that decreased CH4 production. The findings from this study may help to elucidate how PSMs affect rumen bacterial fermentation.
Activity of Salvia dolomitica and Salvia somalensis Essential Oils against Bacteria, Molds and Yeasts.[Pubmed:29438274]
Molecules. 2018 Feb 13;23(2). pii: molecules23020396.
Essential oils (EOs) from Salvia dolomitica and Salvia somalensis, widely employed in the cosmetic and perfume industry, were analyzed for composition and tested against bacterial and fungal pathogens isolated from clinical and environmental specimens. The analyses were carried out against Staphylococcus aureus, Staphylococcus pseudointermedius, Pseudomonas aeruginosa, Escherichia coli, Streptococcus canis, Streptococcus pyogenes, Klebsiella pneumoniae, Proteus mirabilis, Microsporum canis, Microsporum gypseum, Trichophyton mentagrophytes, Aspergillus niger, Aspergillus flavus, Candida albicans, Candida krusei, Mucor sp. and Trichothecium roseum. Both EOs showed similar percentages of total monoterpenes and sesquiterpene hydrocarbons. The main constituents were 1,8-cineole and beta-caryophyllene in S. dolomitica and bornyl acetate and camphor in S. somalensis. The selected EOs have no relevant antifungal or antibacterial activities if compared to conventional drugs.
Comparative Antennal and Behavioral Responses of Summer and Winter Morph Drosophila suzukii (Diptera: Drosophilidae) to Ecologically Relevant Volatiles.[Pubmed:29668908]
Environ Entomol. 2018 Jun 6;47(3):700-706.
Drosophila suzukii (Matsumura) (Diptera: Drosophilidae) is a devastating global pest of berry crops and cherries. Little is understood about its biology during the winter in northern temperate regions, including potential resources that it may utilize during this period. In this study, olfactory and behavioral responses of female D. suzukii to six volatiles (methionol, acetic acid, linalool, bornyl acetate, isoamyl acetate, and geosmin) were evaluated separately for electroantennogram (EAG) and behavioral assays between summer and winter morphs. Results of EAG indicated that isoamyl acetate, acetic acid, and geosmin elicited significantly higher olfactory responses from the antennae of female summer morph D. suzukii compared with those of female winter morph D. suzukii. Winter morph D. suzukii showed reduced antennal response to the volatiles overall. Geosmin and bornyl acetate elicited significantly different behavioral responses from the two morphs in no-choice laboratory behavioral assays. T-maze behavioral assays with geosmin further revealed that summer morphs had a significant aversion, while winter morphs showed no significant aversion to geosmin. Overall, we demonstrate that responses of the two seasonally induced morphs to environmental stimuli are different, and future studies are justified to further understand how these physiological and behavioral differences may contribute to improved pest management of D. suzukii.
Chemical diversity of the essential oils of twenty populations of Tanacetum polycephalum Sch. Bip. from Iran.[Pubmed:29768020]
Nat Prod Res. 2018 May 16:1-4.
Chemical diversity of the essential oils of twenty wild populations of Tanacetum polycephalum Sch. Bip., was investigated. The aerial parts of T. polycephalum were collected at full flowering stage from West Azerbaijan Province of Iran, air-dried; hydrodistilled to produce essential oils. The essential oils were analyzed by GC-FID and GC-MS. A total of forty compounds were identified accounting for 96.4-99.9% of the total oils. The most principal compounds were cis-thujone (0-82.3%), trans-thujone (0-79.8%), camphor (1.3-75.0%), 1,8-cineole (4.5-43.3%), borneol (1.0-36.2%) and bornyl acetate (0-26.8%). Hierarchical cluster analysis based on the percentages (>0.5%) of the essential oils components was carried out to determine the chemical diversity among the populations studied. The cluster analysis resulted in the identification of four main chemotypes namely: 'camphor + 1,8-cineole', 'mixed', 'cis-thujone' and 'trans-thujone'.
Thymus mastichina L. essential oils from Murcia (Spain): Composition and antioxidant, antienzymatic and antimicrobial bioactivities.[Pubmed:29304179]
PLoS One. 2018 Jan 5;13(1):e0190790.
The compositions of essential oils (EOs) from Spanish marjoram (Thymus mastichina L.) grown in several bioclimatic zones of Murcia (SE Spain) were studied to determine their absolute and relative concentrations using gas chromatography-mass spectrometry. 1,8-Cineole and linalool were the main components, followed by alpha-pinene, beta-pinene and alpha-terpineol. (-)-Linalool, (+)-alpha-terpineol and (+)-alpha-pinene were the most abundant enantiomers. When the antioxidant capacities of T. mastichina EOs and their compounds were measured by five methods, EOs and linalool, linalyl acetate, alpha-terpinene and gamma-terpinene, among others, showed antioxidant activities. All four T. mastichina EOs inhibited both lipoxygenase and acetylcholinesterase activities, and they might be useful for further research into inflammatory and Alzheimer diseases. Bornyl acetate and limonene showed the highest lipoxygenase inhibition and 1,8-cineole was the best acetylcholinesterase inhibitor. Moreover, these EOs inhibited the growth of Escherichia coli, Staphylococcus aureus and Candida albicans due to the contribution of their individual compounds. The results underline the potential use of these EOs in manufactured products, such as foodstuff, cosmetics and pharmaceuticals.


