3,4-DimethoxyphenolCAS# 2033-89-8 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 2033-89-8 | SDF | Download SDF |
| PubChem ID | 16251 | Appearance | Oil |
| Formula | C8H10O3 | M.Wt | 154.2 |
| Type of Compound | Phenols | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | 3,4-dimethoxyphenol | ||
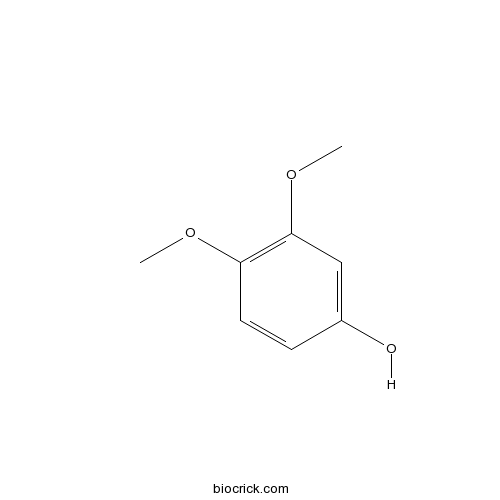
| SMILES | COC1=C(C=C(C=C1)O)OC | ||
| Standard InChIKey | SMFFZOQLHYIRDA-UHFFFAOYSA-N | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | 3,4-Dimethoxyphenol has antimicrobial activity; it also has inhibition of melanin synthesis, it may be used as whitening agents in cosmetics. |
| Targets | Antifection |
| In vitro | 5,6-Dimethoxybenzofuran-3-one derivatives: a novel series of dual Acetylcholinesterase/Butyrylcholinesterase inhibitors bearing benzyl pyridinium moiety.[Pubmed: 23445881]Daru. 2013 Feb 27;21(1):15.
Several studies have been focused on design and synthesis of multi-target anti Alzheimer compounds. Utilizing of the dual Acetylcholinesterase/Butyrylcholinesterase inhibitors has gained more interest to treat the Alzheimer's disease. As a part of a research program to find a novel drug for treating Alzheimer disease, we have previously reported 6-alkoxybenzofuranone derivatives as potent acetylcholinesterase inhibitors. In continuation of our work, we would like to report the synthesis of 5,6-dimethoxy benzofuranone derivatives bearing a benzyl pyridinium moiety as dual Acetylcholinesterase/Butyrylcholinesterase inhibitors.
The antimicrobial activity of Aspergillus fumigatus is enhanced by a pool of bacteria.[Pubmed: 12398291 ]Microbiol Res. 2002;157(3):207-11.
|
| Kinase Assay | Synthesis and cosmetic whitening effect of glycosides derived from several phenylpropanoids.[Pubmed: 16508241]Yakugaku Zasshi. 2006 Mar;126(3):173-7.Plant-derived phenylpropanoid compounds (4-ethyl-2-methoxyphenol, 2,6-dimethoxyphenol, 2,3-dimethoxyphenol, 3,4-Dimethoxyphenol, 3,5-dimethoxyphenol, 3,4-dihydroxycinnamic acid, 4-hydroxy-3-methoxycinnamic acid, and 3-hydroxy-4-methoxycinnamic acid) were glycosidated to form glycoside compounds. |

3,4-Dimethoxyphenol Dilution Calculator

3,4-Dimethoxyphenol Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 6.4851 mL | 32.4254 mL | 64.8508 mL | 129.7017 mL | 162.1271 mL |
| 5 mM | 1.297 mL | 6.4851 mL | 12.9702 mL | 25.9403 mL | 32.4254 mL |
| 10 mM | 0.6485 mL | 3.2425 mL | 6.4851 mL | 12.9702 mL | 16.2127 mL |
| 50 mM | 0.1297 mL | 0.6485 mL | 1.297 mL | 2.594 mL | 3.2425 mL |
| 100 mM | 0.0649 mL | 0.3243 mL | 0.6485 mL | 1.297 mL | 1.6213 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- 3,4,5-Trimethoxy-trans-cinnamic acid
Catalog No.:BCN3423
CAS No.:20329-98-0
- 3,5-Diacetamido-4-methylbenzoic acid
Catalog No.:BCN1505
CAS No.:6633-37-0
- Solamarine
Catalog No.:BCN3806
CAS No.:20318-30-3
- Tiliroside
Catalog No.:BCN4889
CAS No.:20316-62-5
- Procyanidin B1
Catalog No.:BCN6314
CAS No.:20315-25-7
- Solamargine
Catalog No.:BCN2305
CAS No.:20311-51-7
- Saponarin
Catalog No.:BCN2280
CAS No.:20310-89-8
- Clofazimine
Catalog No.:BCC4651
CAS No.:2030-63-9
- Aporheine
Catalog No.:BCN4802
CAS No.:2030-53-7
- NF 279
Catalog No.:BCC6964
CAS No.:202983-32-2
- NF 340
Catalog No.:BCC7785
CAS No.:202982-98-7
- Conantokin-R
Catalog No.:BCC5980
CAS No.:202925-60-8
- H-D-Arg-NH2.2HCl
Catalog No.:BCC2870
CAS No.:203308-91-2
- Daphnoretin
Catalog No.:BCN2473
CAS No.:2034-69-7
- 7-Oxo-beta-sitosterol
Catalog No.:BCN4891
CAS No.:2034-74-4
- Luteollin 5-glucoside
Catalog No.:BCN5391
CAS No.:20344-46-1
- 18-Norabieta-8,11,13-triene-4,15-diol
Catalog No.:BCN1504
CAS No.:203455-81-6
- SNX 482
Catalog No.:BCC5952
CAS No.:203460-30-4
- (+)-Bornyl acetate
Catalog No.:BCN8317
CAS No.:20347-65-3
- (-)-Maackiain
Catalog No.:BCN4892
CAS No.:2035-15-6
- Brefeldin A
Catalog No.:BCC4387
CAS No.:20350-15-6
- 2,4,6,6-Tetramethyl-3(6H)-pyridinone
Catalog No.:BCN4893
CAS No.:203524-64-5
- DMNB
Catalog No.:BCC7259
CAS No.:20357-25-9
- Hastacine
Catalog No.:BCN2086
CAS No.:20361-77-7
Synthesis and cosmetic whitening effect of glycosides derived from several phenylpropanoids.[Pubmed:16508241]
Yakugaku Zasshi. 2006 Mar;126(3):173-7.
Plant-derived phenylpropanoid compounds (4-ethyl-2-methoxyphenol, 2,6-dimethoxyphenol, 2,3-dimethoxyphenol, 3,4-Dimethoxyphenol, 3,5-dimethoxyphenol, 3,4-dihydroxycinnamic acid, 4-hydroxy-3-methoxycinnamic acid, and 3-hydroxy-4-methoxycinnamic acid) were glycosidated to form glycoside compounds. We evaluated the effects of these compounds on the inhibition of tyrosinase and melanin synthesis and their cytotoxicity from the viewpoint of their use as whitening agents in cosmetics. Some compounds had more potent tyrosinase-inhibiting activity than commercial arbutin, which was used as a control, and showed no cytotoxicity at low concentration ranges.
The antimicrobial activity of Aspergillus fumigatus is enhanced by a pool of bacteria.[Pubmed:12398291]
Microbiol Res. 2002;157(3):207-11.
In a screening program for new antibiotic producers, a strain of Aspergillus fumigatus was isolated from Brazilian soil samples. A pool of autoclaved bacteria was added to part of the fungus culture on the second day of fermentation to increase antibiotic production. The chloroform extract from the culture broth to which the pool of autoclaved bacteria was added showed an increase of 55%, 63% and more than 100% in activity against Staphylococcus aureus, Candida albicans and Micrococcus luteus, respectively. Also, the HPLC chromatographic profiles of the chloroform extracts from both culture conditions were different. Two active compounds were isolated from the broth of the culture grown in the presence of pooled bacteria and were identified as 3,4-Dimethoxyphenol and 1,3,5-trimethoxybenzene.
5,6-Dimethoxybenzofuran-3-one derivatives: a novel series of dual Acetylcholinesterase/Butyrylcholinesterase inhibitors bearing benzyl pyridinium moiety.[Pubmed:23445881]
Daru. 2013 Feb 27;21(1):15.
BACKGROUND: Several studies have been focused on design and synthesis of multi-target anti Alzheimer compounds. Utilizing of the dual Acetylcholinesterase/Butyrylcholinesterase inhibitors has gained more interest to treat the Alzheimer's disease. As a part of a research program to find a novel drug for treating Alzheimer disease, we have previously reported 6-alkoxybenzofuranone derivatives as potent acetylcholinesterase inhibitors. In continuation of our work, we would like to report the synthesis of 5,6-dimethoxy benzofuranone derivatives bearing a benzyl pyridinium moiety as dual Acetylcholinesterase/Butyrylcholinesterase inhibitors. METHODS: The synthesis of target compounds was carried out using a conventional method. Bayer-Villiger oxidation of 3,4-dimethoxybenzaldehyde furnished 3,4-Dimethoxyphenol. The reaction of 3,4-Dimethoxyphenol with chloroacetonitrile followed by treatment with HCl solution and then ring closure yielded the 5,6-dimethoxy benzofuranone. Condensation of the later compound with pyridine-4-carboxaldehyde and subsequent reaction with different benzyl halides afforded target compounds. The biological activity was measured using standard Ellman's method. Docking studies were performed to get better insight into interaction of compounds with receptor. RESULTS: The in vitro anti acetylcholinesterase/butyrylcholinesterase activity of compounds revealed that, all of the target compounds have good inhibitory activity against both Acetylcholinesterase/Butyrylcholinesterase enzymes in which compound 5b (IC50 = 52 +/- 6.38nM) was the most active compound against acetylcholinesterase. The same binding mode and interactions were observed for the reference drug donepezil and compound 5b in docking study. CONCLUSIONS: In this study, we presented a new series of benzofuranone-based derivatives having pyridinium moiety as potent dual acting Acetylcholinesterase/Butyrylcholinesterase inhibitors.


