SaponarinCAS# 20310-89-8 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 20310-89-8 | SDF | Download SDF |
| PubChem ID | 441381 | Appearance | Yellow powder |
| Formula | C27H30O15 | M.Wt | 594.52 |
| Type of Compound | Flavonoids | Storage | Desiccate at -20°C |
| Synonyms | Isovitexin-7-O-Glucoside; Saponaretin-7-O-Glucoside; Isovitexin-7-O-Beta-D-Glucopyranoside | ||
| Solubility | Sparingly soluble in methanol and water | ||
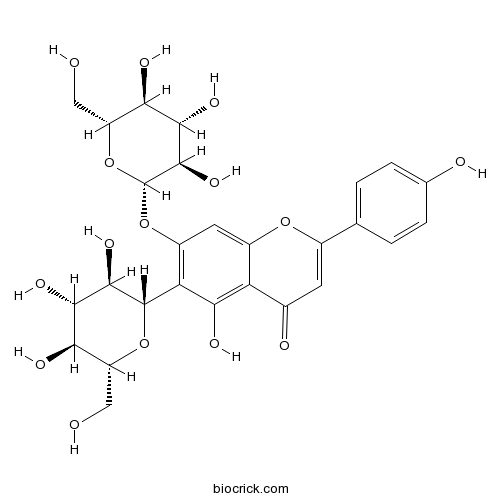
| Chemical Name | 5-hydroxy-2-(4-hydroxyphenyl)-6-[(2S,3R,4R,5S,6R)-3,4,5-trihydroxy-6-(hydroxymethyl)oxan-2-yl]-7-[(2S,3R,4R,5S,6R)-3,4,5-trihydroxy-6-(hydroxymethyl)oxan-2-yl]oxychromen-4-one | ||
| SMILES | C1=CC(=CC=C1C2=CC(=O)C3=C(C(=C(C=C3O2)OC4C(C(C(C(O4)CO)O)O)O)C5C(C(C(C(O5)CO)O)O)O)O)O | ||
| Standard InChIKey | HGUVPEBGCAVWID-KETMJRJWSA-N | ||
| Standard InChI | InChI=1S/C27H30O15/c28-7-15-19(32)22(35)24(37)26(40-15)18-14(41-27-25(38)23(36)20(33)16(8-29)42-27)6-13-17(21(18)34)11(31)5-12(39-13)9-1-3-10(30)4-2-9/h1-6,15-16,19-20,22-30,32-38H,7-8H2/t15-,16-,19-,20-,22+,23+,24-,25-,26+,27-/m1/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | 1. Saponarin, which isolated from Gypsophila trichotoma Wend, shows in vitro and in vivo hepatoprotective and antioxidant activity against CCl4-induced liver damage. 2. Saponarin has antioxidant activity against cocaine-induced oxidative stress and hepatotoxicity. 3. Saponarin exerts anti-inflammatory effects in LPS-induced RAW 264.7 macrophages via inhibition of NF-κB, ERK and p38 signaling. 4. Saponarin shows hypoglycemic activity in the range of 20-80 mg/kg compared to 100-200 mg/kg for acarbose as reported. 5. Saponarin is characterized as α-glucosidase inhibitor present in Tinospora cordifolia, it also has hypoglycemic activity. 6. Saponarin exerts slight antihypertensive activity in non-diabetic spontaneously hypertensive rats (SHR), however, such effect is not observed in streptozotocin-induced diabetic SHR (SHR-D), indicates that diabetes and hypertension in combination are more difficult to be modulated by saponarin. |
| Targets | P450 (e.g. CYP17) | NF-kB | ERK | IL Receptor | p38MAPK | COX |

Saponarin Dilution Calculator

Saponarin Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 1.682 mL | 8.4101 mL | 16.8203 mL | 33.6406 mL | 42.0507 mL |
| 5 mM | 0.3364 mL | 1.682 mL | 3.3641 mL | 6.7281 mL | 8.4101 mL |
| 10 mM | 0.1682 mL | 0.841 mL | 1.682 mL | 3.3641 mL | 4.2051 mL |
| 50 mM | 0.0336 mL | 0.1682 mL | 0.3364 mL | 0.6728 mL | 0.841 mL |
| 100 mM | 0.0168 mL | 0.0841 mL | 0.1682 mL | 0.3364 mL | 0.4205 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Clofazimine
Catalog No.:BCC4651
CAS No.:2030-63-9
- Aporheine
Catalog No.:BCN4802
CAS No.:2030-53-7
- NF 279
Catalog No.:BCC6964
CAS No.:202983-32-2
- NF 340
Catalog No.:BCC7785
CAS No.:202982-98-7
- Conantokin-R
Catalog No.:BCC5980
CAS No.:202925-60-8
- glucagon receptor antagonists 2
Catalog No.:BCC1594
CAS No.:202917-18-8
- glucagon receptor antagonists 3
Catalog No.:BCC1595
CAS No.:202917-17-7
- 7-Benzyloxyindole
Catalog No.:BCC8778
CAS No.:20289-27-4
- 4-Benzyloxyindole
Catalog No.:BCC8700
CAS No.:20289-26-3
- 8-Hydroxy-3,5,7,3',4',5'-hexamethoxyflavone
Catalog No.:BCN1506
CAS No.:202846-95-5
- Rosmarinic acid
Catalog No.:BCN5893
CAS No.:20283-92-5
- Safinamide Mesylate
Catalog No.:BCC2320
CAS No.:202825-46-5
- Solamargine
Catalog No.:BCN2305
CAS No.:20311-51-7
- Procyanidin B1
Catalog No.:BCN6314
CAS No.:20315-25-7
- Tiliroside
Catalog No.:BCN4889
CAS No.:20316-62-5
- Solamarine
Catalog No.:BCN3806
CAS No.:20318-30-3
- 3,5-Diacetamido-4-methylbenzoic acid
Catalog No.:BCN1505
CAS No.:6633-37-0
- 3,4,5-Trimethoxy-trans-cinnamic acid
Catalog No.:BCN3423
CAS No.:20329-98-0
- 3,4-Dimethoxyphenol
Catalog No.:BCN4890
CAS No.:2033-89-8
- H-D-Arg-NH2.2HCl
Catalog No.:BCC2870
CAS No.:203308-91-2
- Daphnoretin
Catalog No.:BCN2473
CAS No.:2034-69-7
- 7-Oxo-beta-sitosterol
Catalog No.:BCN4891
CAS No.:2034-74-4
- Luteollin 5-glucoside
Catalog No.:BCN5391
CAS No.:20344-46-1
- 18-Norabieta-8,11,13-triene-4,15-diol
Catalog No.:BCN1504
CAS No.:203455-81-6
Hepatoprotective effects of saponarin, isolated from Gypsophila trichotoma Wend. on cocaine-induced oxidative stress in rats.[Pubmed:21722413]
Redox Rep. 2011;16(2):56-61.
The antioxidant effect of Saponarin, which is the main flavone isolated from Gypsophila trichotoma Wend., and its protection against cocaine hepatotoxicity were investigated in male Wistar rats. The animals were treated with cocaine (40 mg/kg i.p.) alone and also after 3 consecutive days of pretreatment with Saponarin (80 mg/kg p.o.). After 18 hours the rats were sacrificed by decapitation. The production of thiobarbituric acid reactive substances, reduced glutathione (GSH) and the activity of the following antioxidant enzymes: catalase, superoxide dismutase, glutathione peroxidase, glutathione reductase, and glutathione-S-transferase were assessed in liver homogenate. Administered alone, cocaine induced significant hepatotoxicity manifested with GSH depletion and reduced antioxidant defences. Saponarin pretreatment, however, decreased cocaine toxicity both by increasing GSH levels and antioxidant enzyme activities. The results of this study proved the antioxidant activity of Saponarin and its protective effect against cocaine-induced oxidative stress and hepatotoxicity.
Hepatoprotective and antioxidant effects of saponarin, isolated from Gypsophila trichotoma Wend. on paracetamol-induced liver damage in rats.[Pubmed:23878818]
Biomed Res Int. 2013;2013:757126.
The hepatoprotective potential of Saponarin, isolated from Gypsophila trichotoma, was evaluated in vitro/in vivo using a hepatotoxicity model of paracetamol-induced liver injury. In freshly isolated rat hepatocytes, paracetamol (100 mu mol) led to a significant decrease in cell viability, increased LDH leakage, decreased levels of cellular GSH, and elevated MDA quantity. Saponarin (60-0.006 mu g/mL) preincubation, however, significantly ameliorated paracetamol-induced hepatotoxicity in a concentration-dependent manner. The beneficial effect of Saponarin was also observed in vivo. Rats were challenged with paracetamol alone (600 mg/kg, i.p.) and after 7-day pretreatment with Saponarin (80 mg/kg, oral gavage). Paracetamol toxicity was evidenced by increase in MDA quantity and decrease in cell GSH levels and antioxidant defence system. No changes in phase I enzyme activities of AH and EMND and cytochrome P 450 quantity were detected. Saponarin pretreatment resulted in significant increase in cell antioxidant defence system and GSH levels and decrease in lipid peroxidation. The biochemical changes are in good correlation with the histopathological data. Protective activity of Saponarin was similar to the activity of positive control silymarin. On the basis of these results, it can be concluded that Saponarin exerts antioxidant and hepatoprotective activity against paracetamol liver injury in vitro/in vivo.
Hypoglycemic activity of the antioxidant saponarin, characterized as alpha-glucosidase inhibitor present in Tinospora cordifolia.[Pubmed:18951283]
J Enzyme Inhib Med Chem. 2009 Jun;24(3):684-90.
Tinospora cordifolia, used in anti-diabetic herbal drug preparations, was reported [12] to contain an alpha-glucosidase inhibitor, characterized as Saponarin (apigenin-6-C-glucosyl-7-O-glucoside). The leaf extract had appreciable antioxidant and hydroxyl radical scavenging activities and contained the flavonoid in the range of 32.1 +/- 1.5-45.5 +/- 3.5 mg/g of dry solid. Saponarin showed mixed competitive inhibition on activities of alpha-glucosidase and sucrase of different origins. IC(50), Ki and ki' values determined were 48 muM, 8 muM and 19.5 microM respectively for intestinal maltase and 35 microM, 6 microM and 13 microM respectively for intestinal sucrase. When given orally to maltose-fed rat, Saponarin showed hypoglycemic activity in the range of 20-80 mg/kg compared to 100-200 mg/kg for acarbose as reported.
Protective effects of the apigenin-O/C-diglucoside saponarin from Gypsophila trichotoma on carbone tetrachloride-induced hepatotoxicity in vitro/in vivo in rats.[Pubmed:24011529]
Phytomedicine. 2014 Jan 15;21(2):148-54.
This study investigated the hepatoprotective activity of Saponarin, isolated from Gypsophila trichotoma Wend., using in vitro/in vivo hepatotoxicity model based on carbone tetrachloride (CCl(4))-induced liver damage in male Wistar rats. The effect of Saponarin was compared with those of silymarin. In vitro experiments were carried out in primary isolated rat hepatocytes. Cell incubation with CCl(4) (86 mumol l(-)(1)) led to a significant decrease in cell viability, increased LDH leakage, decreased levels of cellular GSH and elevation in MDA quantity. Cell pre-incubation with Saponarin (60-0.006 mug/ml) significantly ameliorated CCl(4)-induced hepatic damage in a concentration-dependent manner. These results were supported by the following in vivo study. Along with decreased MDA quantity and increased level of cell protector GSH, seven day pre-treatment of rats with Saponarin (80 mg/kg bw; p.o.) also prevented CCl(4) (10%, p.o.)-caused oxidative damage by increasing antioxidant enzyme activities (CAT, SOD, GST, GPx, GR). Biotransformation phase I enzymes were also assessed. Administered alone, Saponarin decreased EMND and AH activities but not at the same extent as CCl(4) did. However, pre-treatment with Saponarin significantly increased enzyme activities in comparison to CCl(4) only group. The observed biochemical changes were consistent with histopathological observations where the hepatoprotective effect of Saponarin was comparative to the effects of the known hepatoprotecor silymarin. Our results suggest that Saponarin, isolated from Gypsophila trichotoma Wend., showed in vitro and in vivo hepatoprotective and antioxidant activity against CCl(4)-induced liver damage.
Antidiabetic and antioxidant effects of saponarin from Gypsophila trichotoma on streptozotocin-induced diabetic normotensive and hypertensive rats.[Pubmed:27064007]
Phytomedicine. 2016 May 15;23(5):483-90.
BACKGROUND: Diabetes and hypertension are diseases that often coexist, which increases the risk of chronic organ damages and cardiovascular complications. PURPOSE: To evaluate the effects of Saponarin, isolated from Gypsophila trichotoma Wend, on blood pressure, glycemia, body weight, and liver biochemical parameters related to oxidative stress in diabetic normotensive Wistar Kyoto rats (NTR) and spontaneously hypertensive rats (SHR). METHODS: Diabetes was induced by administration of streptozotocin (40 mg/kg, i.p.). The following biochemical parameters: reduced glutathione (GSH), malondialdehyde (MDA), total cytochrome P450, aniline hydroxylase (AH) activity, as well as the activities of antioxidant enzymes such as glutathione peroxidase (GPx), glutathione reductase (GR) and glutathione S-transferase (GST) were measured in the livers of euthanized rats. RESULTS: Saponarin exerted slight antihypertensive activity in non-diabetic SHR, judged by 19% (p<0.05) decrease of the initial blood pressure. However, such effect was not observed in streptozotocin-induced diabetic SHR (SHR-D). Streptozotocin-induced diabetes was evidenced by 78% (p<0.05) and by 171% (p<0.05) increase in blood glucose level in NTR and SHR, respectively. In non-diabetic SHR the initial MDA quantity was by 36% (p<0.05) higher and the initial GSH levels were by 28% (p<0.05) lower in comparison to non-diabetic NTR. Significant decrease in the activities of GPx, GR, and GST was measured in the livers of all diabetic rats. Treatment with Saponarin ameliorated the above mentioned liver parameters in both diabetic strains, however its effects were less pronounced in the diabetic SHR group. CONCLUSION: Taken together our data indicate that diabetes and hypertension in combination are more difficult to be modulated by Saponarin.
Saponarin from barley sprouts inhibits NF-kappaB and MAPK on LPS-induced RAW 264.7 cells.[Pubmed:25238253]
Food Funct. 2014 Nov;5(11):3005-13.
Saponarin (SA), a natural flavonoid, is known for its antioxidant and hepatoprotective activities. SA is the predominant compound (1142.7 +/- 0.9 mg per 100 g) in barley sprouts, constituting 72% of the total polyphenol content. We investigated, for the first time, the effects of SA from barley sprouts on cellular anti-inflammatory responses. In lipopolysaccharide (LPS)-induced RAW 264.7 macrophages, SA suppressed the activation of NF-kappaB, as evidenced by the inhibition of NF-kappaB DNA binding, nuclear translocation, IkappaBalpha phosphorylation, and reporter gene expression, and it downregulated the expression of the pro-inflammatory mediator IL-6. Furthermore, SA reduced the transcription of NF-kappaB target molecules COX2 and FLIP inhibited the phosphorylation of mitogen-activated protein kinases ERK and p38. These results suggest that SA isolated from barley sprouts exerts anti-inflammatory effects in LPS-induced RAW 264.7 macrophages via inhibition of NF-kappaB, ERK and p38 signaling. Thus, SA may be a promising natural anti-inflammatory agent.


