7-BenzyloxyindoleCAS# 20289-27-4 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 20289-27-4 | SDF | Download SDF |
| PubChem ID | 260798 | Appearance | Powder |
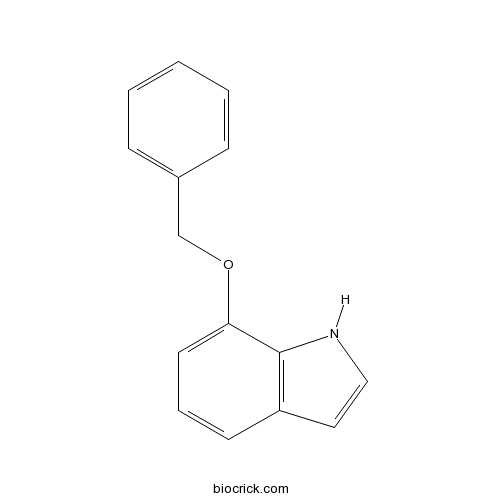
| Formula | C15H13NO | M.Wt | 223.3 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | 7-phenylmethoxy-1H-indole | ||
| SMILES | C1=CC=C(C=C1)COC2=CC=CC3=C2NC=C3 | ||
| Standard InChIKey | DIGZMTAFOACVBW-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C15H13NO/c1-2-5-12(6-3-1)11-17-14-8-4-7-13-9-10-16-15(13)14/h1-10,16H,11H2 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

7-Benzyloxyindole Dilution Calculator

7-Benzyloxyindole Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 4.4783 mL | 22.3914 mL | 44.7828 mL | 89.5656 mL | 111.957 mL |
| 5 mM | 0.8957 mL | 4.4783 mL | 8.9566 mL | 17.9131 mL | 22.3914 mL |
| 10 mM | 0.4478 mL | 2.2391 mL | 4.4783 mL | 8.9566 mL | 11.1957 mL |
| 50 mM | 0.0896 mL | 0.4478 mL | 0.8957 mL | 1.7913 mL | 2.2391 mL |
| 100 mM | 0.0448 mL | 0.2239 mL | 0.4478 mL | 0.8957 mL | 1.1196 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- 4-Benzyloxyindole
Catalog No.:BCC8700
CAS No.:20289-26-3
- 8-Hydroxy-3,5,7,3',4',5'-hexamethoxyflavone
Catalog No.:BCN1506
CAS No.:202846-95-5
- Rosmarinic acid
Catalog No.:BCN5893
CAS No.:20283-92-5
- Safinamide Mesylate
Catalog No.:BCC2320
CAS No.:202825-46-5
- Ralfinamide mesylate
Catalog No.:BCC7844
CAS No.:202825-45-4
- BMS 191011
Catalog No.:BCC7448
CAS No.:202821-81-6
- Licoagrochalcone A
Catalog No.:BCC8197
CAS No.:202815-28-9
- Orexin B (mouse)
Catalog No.:BCC5766
CAS No.:202801-92-1
- 4-Methoxycoumarine
Catalog No.:BCN6536
CAS No.:20280-81-3
- Homodihydrocapsaicin I
Catalog No.:BCN7844
CAS No.:20279-06-5
- Forsythenside A
Catalog No.:BCN6440
CAS No.:202721-09-3
- Nicotinamide N-oxide
Catalog No.:BCN1969
CAS No.:1986-81-8
- glucagon receptor antagonists 3
Catalog No.:BCC1595
CAS No.:202917-17-7
- glucagon receptor antagonists 2
Catalog No.:BCC1594
CAS No.:202917-18-8
- Conantokin-R
Catalog No.:BCC5980
CAS No.:202925-60-8
- NF 340
Catalog No.:BCC7785
CAS No.:202982-98-7
- NF 279
Catalog No.:BCC6964
CAS No.:202983-32-2
- Aporheine
Catalog No.:BCN4802
CAS No.:2030-53-7
- Clofazimine
Catalog No.:BCC4651
CAS No.:2030-63-9
- Saponarin
Catalog No.:BCN2280
CAS No.:20310-89-8
- Solamargine
Catalog No.:BCN2305
CAS No.:20311-51-7
- Procyanidin B1
Catalog No.:BCN6314
CAS No.:20315-25-7
- Tiliroside
Catalog No.:BCN4889
CAS No.:20316-62-5
- Solamarine
Catalog No.:BCN3806
CAS No.:20318-30-3
Efficacy of 7-benzyloxyindole and other halogenated indoles to inhibit Candida albicans biofilm and hyphal formation.[Pubmed:29656577]
Microb Biotechnol. 2018 Nov;11(6):1060-1069.
Certain pathogenic bacteria and yeast form biofilms on biotic and abiotic surfaces including medical devices and implants. Hence, the development of antibiofilm coating materials becomes relevant. The virulence of those colonizing pathogens can be reduced by inhibiting biofilm formation rather than killing pathogens using excessive amounts of antimicrobials, which is touted as one of the main reasons for the development of drug resistance. Candida albicans is an opportunistic fungal pathogen, and the transition of yeast cells to hyphal cells is believed to be a crucial virulence factor. Previous studies have shown that indole and its derivatives possess antivirulence properties against various bacterial pathogens. In this study, we used various indole derivatives to investigate biofilm-inhibiting activity against C. albicans. Our study revealed that 7-Benzyloxyindole, 4-fluoroindole and 5-iodoindole effectively inhibited biofilm formation compared to the antifungal agent fluconazole. Particularly, 7-Benzyloxyindole at 0.02 mM (4.5 mug ml(-1) ) significantly reduced C. albicans biofilm formation, but had no effect on planktonic cells, and this finding was confirmed by a 2,3-bis-(2-methoxy-4-nitro-5-sulfophenyl)-2H-tetrazolium-5-carboxanilide (XTT) assay and three-dimensional confocal laser scanning microscopy. Scanning electron microscopy analyses revealed that 7-Benzyloxyindole effectively inhibited hyphal formation, which explains biofilm inhibition. Transcriptomic analysis showed that 7-Benzyloxyindole downregulated the expressions of several hypha/biofilm-related genes (ALS3, ECE1, HWP1 and RBT1). A C. albicans-infected Caenorhabditis elegans model system was used to confirm the antivirulence efficacy of 7-Benzyloxyindole.
Indole and 7-benzyloxyindole attenuate the virulence of Staphylococcus aureus.[Pubmed:23318836]
Appl Microbiol Biotechnol. 2013 May;97(10):4543-52.
Human pathogens can readily develop drug resistance due to the long-term use of antibiotics that mostly inhibit bacterial growth. Unlike antibiotics, antivirulence compounds diminish bacterial virulence without affecting cell viability and thus, may not lead to drug resistance. Staphylococcus aureus is a major agent of nosocomial infections and produces diverse virulence factors, such as the yellow carotenoid staphyloxanthin, which promotes resistance to reactive oxygen species (ROS) and the host immune system. To identify novel antivirulence compounds, bacterial signal indole present in animal gut and diverse indole derivatives were investigated with respect to reducing staphyloxanthin production and the hemolytic activity of S. aureus. Treatment with indole or its derivative 7-Benzyloxyindole (7BOI) caused S. aureus to become colorless and inhibited its hemolytic ability without affecting bacterial growth. As a result, S. aureus was more easily killed by hydrogen peroxide (H(2)O(2)) and by human whole blood in the presence of indole or 7BOI. In addition, 7BOI attenuated S. aureus virulence in an in vivo model of nematode Caenorhabditis elegans, which is readily infected and killed by S. aureus. Transcriptional analyses showed that both indole and 7BOI repressed the expressions of several virulence genes such as alpha-hemolysin gene hla, enterotoxin seb, and the protease genes splA and sspA and modulated the expressions of the important regulatory genes agrA and sarA. These findings show that indole derivatives are potential candidates for use in antivirulence strategies against persistent S. aureus infection.


