CL 218872Benzodiazepine agonist CAS# 66548-69-4 |
- Narciclasine
Catalog No.:BCN4732
CAS No.:29477-83-6
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 66548-69-4 | SDF | Download SDF |
| PubChem ID | 107950 | Appearance | Powder |
| Formula | C13H9F3N4 | M.Wt | 278.24 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble to 50 mM in DMSO and to 25 mM in ethanol | ||
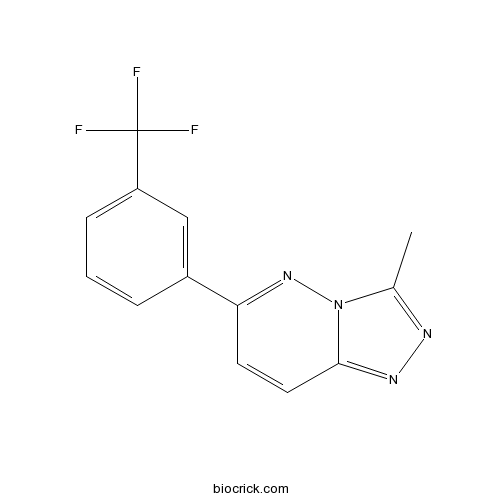
| Chemical Name | 3-methyl-6-[3-(trifluoromethyl)phenyl]-[1,2,4]triazolo[4,3-b]pyridazine | ||
| SMILES | CC1=NN=C2N1N=C(C=C2)C3=CC(=CC=C3)C(F)(F)F | ||
| Standard InChIKey | GUOQUXNJZHGPQF-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C13H9F3N4/c1-8-17-18-12-6-5-11(19-20(8)12)9-3-2-4-10(7-9)13(14,15)16/h2-7H,1H3 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Benzodiazepine agonist displaying selectivity for α1 subunit-containing GABAA receptors (Ki values are 130, 1820, 1530, > 10000, 490 and > 10000 nM for α1, α2, α3, α4, α5 and α6-subunit containing receptors respectively). Orally active anxiolytic and anticonvulsant in vivo. |

CL 218872 Dilution Calculator

CL 218872 Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.594 mL | 17.9701 mL | 35.9402 mL | 71.8804 mL | 89.8505 mL |
| 5 mM | 0.7188 mL | 3.594 mL | 7.188 mL | 14.3761 mL | 17.9701 mL |
| 10 mM | 0.3594 mL | 1.797 mL | 3.594 mL | 7.188 mL | 8.985 mL |
| 50 mM | 0.0719 mL | 0.3594 mL | 0.7188 mL | 1.4376 mL | 1.797 mL |
| 100 mM | 0.0359 mL | 0.1797 mL | 0.3594 mL | 0.7188 mL | 0.8985 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Augustifolin
Catalog No.:BCN3232
CAS No.:66548-01-4
- Ansamitocin P-3
Catalog No.:BCN8373
CAS No.:66547-09-9
- Propacetamol hydrochloride
Catalog No.:BCC9129
CAS No.:66532-86-3
- Benzoin isopropyl ether
Catalog No.:BCC8856
CAS No.:6652-28-4
- MSOP
Catalog No.:BCC6801
CAS No.:66515-29-5
- Bicifadine hydrochloride
Catalog No.:BCC7925
CAS No.:66504-75-4
- Amantadine HCl
Catalog No.:BCC4465
CAS No.:665-66-7
- JW 55
Catalog No.:BCC2453
CAS No.:664993-53-7
- H-D-Lys(Boc)-OMe.HCl
Catalog No.:BCC2990
CAS No.:66494-53-9
- Kaempferol 3-O-(6''-O-acetyl)glucoside-7-O-rhamnoside
Catalog No.:BCN1385
CAS No.:66465-24-5
- 2-(2,4-Diaminophenoxy)ethanol dihydrochloride
Catalog No.:BCN8497
CAS No.:66422-95-5
- 6-Amino-1,3-dimethyluracil
Catalog No.:BCC8755
CAS No.:6642-31-5
- ent-3beta-Hydroxykaur-16-en-19-oic acid
Catalog No.:BCN6472
CAS No.:66556-91-0
- Tsugafolin
Catalog No.:BCN4026
CAS No.:66568-97-6
- Forskolin
Catalog No.:BCN2332
CAS No.:66575-29-9
- Dihydrorotenone
Catalog No.:BCN2726
CAS No.:6659-45-6
- Agaric acid
Catalog No.:BCC9216
CAS No.:666-99-9
- Boc-Phenylalaninol
Catalog No.:BCC2718
CAS No.:66605-57-0
- RU 24969
Catalog No.:BCC5423
CAS No.:66611-26-5
- Risperidone hydrochloride
Catalog No.:BCC4205
CAS No.:666179-74-4
- Risperidone mesylate
Catalog No.:BCC4206
CAS No.:666179-96-0
- 2',4'-Dihydroxy-3,7':4,8'-diepoxylign-7-ene
Catalog No.:BCN6645
CAS No.:666250-52-8
- GW842166X
Catalog No.:BCC4413
CAS No.:666260-75-9
- Biotin Hydrazide
Catalog No.:BCC3582
CAS No.:66640-86-6
CL 218,872 a triazolopyridazine with a selective affinity for the benzodiazepine BZ1 receptor subtype, retards the development and expression of amygdaloid-kindled seizures: effects of flumazenil.[Pubmed:7902274]
Epilepsy Res. 1993 Sep;16(1):19-26.
To clarify the role of benzodiazepine receptors in kindling, the present experiment assessed the effects of CL 218,872 (1, 5, 10, and 20 mg/kg), a triazolopyridazine with a selective affinity for the putative benzodiazepine BZ1 receptor subtype, on the development and expression of amygdaloid-kindled seizures. Additionally, we assessed the effects of flumazenil (10 mg/kg), a non-specific benzodiazepine receptor antagonist, on kindling and the expression of kindled seizures alone or concomitantly with CL 218,872 (20 mg/kg). CL 218,872 retarded the development of kindled seizures in a linear dose-dependent manner; rats treated with 5, 10, and 20 mg/kg, but not 1 mg/kg, of CL 218,872 required a greater number of afterdischarges (ADs) to develop generalized seizures than controls. Flumazenil also retarded kindling and failed to attenuate the prophylactic effect of CL 218,872. In a cross-over procedure rats that did not develop generalized seizures after 30 ADs while under drug were rekindled under vehicle and rats kindled under vehicle were subsequently tested under drug. Rats crossed over to vehicle rekindled at a faster rate than did controls during initial kindling, suggesting that some kindling had occurred under the drug. CL 218,872 also dose-dependently depressed kindled seizures and this was attenuated by flumazenil, which had little effect on kindled seizures by itself. Together, these data suggest that CL 218,872 is a potent anticonvulsant, implicating the BZ1 receptor subtype in seizure development and in the expression of kindled seizures.
Modulation of the electrically evoked release of 5-[3H]hydroxytryptamine from rat cerebral cortex: effects of alpidem, CL 218872, and diazepam.[Pubmed:2844992]
J Neurochem. 1988 Nov;51(5):1414-21.
The effect of omega (benzodiazepine)-receptor agonists, antagonists, and inverse agonists on the electrically evoked release of 5-[3H]hydroxytryptamine ([3H]5-HT) was studied in superfused slices of the rat frontal cerebral cortex. The electrically evoked release of [3H]5-HT was enhanced by nanomolar concentrations of diazepam and the selective omega 1-receptor agonists alpidem and CL 218872. The omega 1/omega 2- and omega 1-receptor antagonists flumazenil and CGS 8216, respectively, did not modify the electrically evoked release of [3H]5-HT. The omega 3-receptor agonist Ro 5-4864 and the omega 1-receptor inverse agonist ethyl-beta-carboline-3-carboxylate on their own did not affect the electrically evoked release of [3H]5-HT. On the other hand, the inverse agonist 6,7-dimethoxy-4-ethyl-beta-carboline-3-carboxylic acid methyl ester (DMCM), at micromolar concentrations, inhibited both the spontaneous and the evoked release of [3H]5-HT. The facilitation of the electrically evoked release of [3H]5-HT by diazepam, alpidem, or CL 218872 was potentiated by gamma-aminobutyric acid (GABA). Exposure to flumazenil and CGS 8216 antagonized the facilitation by diazepam, alpidem, or CL 218872 of [3H]5-HT release. The inhibition of the release of [3H]5-HT by DMCM was not modified by exposure to either flumazenil, CGS 8216, or GABA. The inhibitory effect of DMCM was not observed when monoamine oxidase activity was inhibited by pargyline.(ABSTRACT TRUNCATED AT 250 WORDS)
Like diazepam, CL 218,872, a selective ligand for the benzodiazepine omega 1 receptor subtype, impairs place learning in the Morris water maze.[Pubmed:1352055]
Psychopharmacology (Berl). 1992;107(2-3):347-51.
The sedative, anxiolytic, and amnesic effects of diazepam were compared to those of CL 218,872, a triazolopyridazine that has a preferential affinity for the benzodiazepine omega 1 receptor subtype. Spontaneous locomotion was assessed using a running wheel, anxiety was assessed using an open-field divided into central and peripheral areas (thigmotaxis), and amnesia was assessed using the Morris water maze. It was found that CL 218,872, like diazepam, depressed spontaneous locomotion, reduced anxiety, and impaired place learning in a dose-dependent manner. Flumazenil, a benzodiazepine receptor antagonist with a similar affinity for both omega 1 and omega 2 subtypes, reversed all of the effects of diazepam and antagonized the anxiolytic and amnesic effects, and some but not all of the sedative effects of CL 218,872. These results suggest that the selective activation of the omega 1 receptor subtype by CL 218,872 is sufficient to produce sedation, anxiolysis, and amnesia in a manner similar to that produced by the coactivation of both the omega 1 and omega 2 receptor subtypes with diazepam.
In vivo benzodiazepine receptor occupancy by CL 218,872 visualized by positron emission tomography in the brain of the living baboon: modulation by GABAergic transmission and relation with anticonvulsant activity.[Pubmed:1673661]
Exp Brain Res. 1991;83(2):397-402.
In vivo benzodiazepine receptor occupancy by increasing doses of CL 218,872 has been evaluated in the baboon Papio papio, using (11C) RO 15-1788 as specific radioligand and positron emission tomography as external detection system. Although BZR heterogeneity has been previously demonstrated in the brain of the living baboon using PET, we did not observe in our studies that CL 218,872 interacts preferentially with one of the BZR subtypes. The monophasic pattern of the dose dependent CL 218,872 displacement curve and the corresponding "in vivo Hill coefficient" near unity suggest that CL 218,872 binds in cerebral baboon cortex with a similar affinity with BZ1 as well as BZ2 subtypes. The anticonvulsant properties of CL 218,872 against bicuculline and allylglycine-induced seizures were correlated with benzodiazepine receptor occupancy by assessment of electroencephalographic activity during positron emission tomography studies. Our data confirmed in vivo the hypothesis of a partial agonist anticonvulsant activity of CL 218,872. At the same time, the use of a GABA-antagonist (bicuculline) or an inhibitor of the GABA synthesis (allylglycine) suggested the existence of an allosteric interaction between benzodiazepine receptors and GABA receptors.
Benzodiazepine receptors: cellular and behavioral characteristics.[Pubmed:40258]
Pharmacol Biochem Behav. 1979 May;10(5):831-43.
Brain specific benzodiazepine receptors appear to mediate the pharmacological properties of benzodiazepines. A neuronal localization for these receptors is suggested by the parallel decrease in the number of benzodiazepine receptors and cerebellar Purkinje cells in "nervous" mutant mice. Electrophysiological results are compatible with an action of benzodiazepines on neuronally localized, physiological receptors. Biochemical, electrophysiological and behavioral experiments highlight the possible importance of frontal cortex in mediating the anxiolytic properties of the benzodiazepines. Triazolenetetrazoles act upon benzodiazepine receptors, increase punished responding and protect against penetylenetetrazole-induced convulsions, but do not produce the side effects associated with benzodiazepines or affect classical neurotransmitter systems. The structural similarities between triazolopyridazines, purines and the indole portion of certain peptides may provide insights into the nature of the endogenous ligand.
Some properties of brain specific benzodiazepine receptors: new evidence for multiple receptors.[Pubmed:40257]
Pharmacol Biochem Behav. 1979 May;10(5):825-30.
Several new lines of evidence suggest the existence of two or more distinct types of benzodiazepine receptors, in contrast to earlier results suggesting the presence of only one class of receptors. Appropriate thermoinactivation experiments indicate two receptors with different thermostabilities. Several triazolopyridazines, with some of the pharmacological properties of anxiolytics have recently been shown to displace 3H-diazepam and 3H-flunitrazepam with Ki values in the 6 to 100 nanomolar range. These new substances are active in conflict tests in rats and monkeys and prevent metrazol induced seizures in vivo, but strikingly lack the ataxia and sedative properties of the benzodiazepines. Hill analyses of dose-response curves for some of these substances yields Hill coefficients in the range of 0.4--0.6, suggesting that these compounds may be able to discriminate between several types of benzodiazepine receptors.


