Copalic acidCAS# 24470-48-2 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 24470-48-2 | SDF | Download SDF |
| PubChem ID | 23786438 | Appearance | Oil |
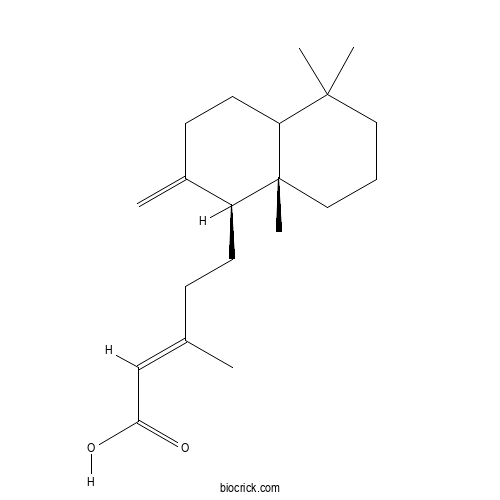
| Formula | C20H32O2 | M.Wt | 304.5 |
| Type of Compound | Diterpenoids | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | (E)-5-[(1S,8aS)-5,5,8a-trimethyl-2-methylidene-3,4,4a,6,7,8-hexahydro-1H-naphthalen-1-yl]-3-methylpent-2-enoic acid | ||
| SMILES | CC(=CC(=O)O)CCC1C(=C)CCC2C1(CCCC2(C)C)C | ||
| Standard InChIKey | JFQBNOIJWROZGE-YSNLAMCCSA-N | ||
| Standard InChI | InChI=1S/C20H32O2/c1-14(13-18(21)22)7-9-16-15(2)8-10-17-19(3,4)11-6-12-20(16,17)5/h13,16-17H,2,6-12H2,1,3-5H3,(H,21,22)/b14-13+/t16-,17?,20+/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Copalic acid Dilution Calculator

Copalic acid Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.2841 mL | 16.4204 mL | 32.8407 mL | 65.6814 mL | 82.1018 mL |
| 5 mM | 0.6568 mL | 3.2841 mL | 6.5681 mL | 13.1363 mL | 16.4204 mL |
| 10 mM | 0.3284 mL | 1.642 mL | 3.2841 mL | 6.5681 mL | 8.2102 mL |
| 50 mM | 0.0657 mL | 0.3284 mL | 0.6568 mL | 1.3136 mL | 1.642 mL |
| 100 mM | 0.0328 mL | 0.1642 mL | 0.3284 mL | 0.6568 mL | 0.821 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Diplacone
Catalog No.:BCX0207
CAS No.:73676-38-7
- 14,15-Dinorcleroda-3,11E-dien-13-one
Catalog No.:BCX0206
CAS No.:1186523-96-5
- Clauszoline B
Catalog No.:BCX0205
CAS No.:185508-03-6
- Kolavenic acid
Catalog No.:BCX0204
CAS No.:25436-90-2
- Crinine
Catalog No.:BCX0203
CAS No.:510-67-8
- 10-Hydroxyoleuropein
Catalog No.:BCX0202
CAS No.:84638-44-8
- Denticulatain E
Catalog No.:BCX0201
CAS No.:1919050-76-2
- Gnetuhainin N
Catalog No.:BCX0200
CAS No.:337464-99-0
- 4α-Hydroxybisabola-2,10-diene-1,9-dione
Catalog No.:BCX0199
CAS No.:1627567-79-6
- Heliobuphthalmin lactone
Catalog No.:BCX0198
CAS No.:580-73-4
- Macowine
Catalog No.:BCX0197
CAS No.:307494-75-3
- Methyl pechueloate
Catalog No.:BCX0196
CAS No.:83161-59-5
- 1-Epideacetylbowdensine
Catalog No.:BCX0209
CAS No.:101219-55-0
- Fibraurin
Catalog No.:BCX0210
CAS No.:25254-84-6
- Pratorimine
Catalog No.:BCX0211
CAS No.:88660-12-2
- Gnetuhainin O
Catalog No.:BCX0212
CAS No.:337464-95-6
- (2R,3S)-Pterosin C
Catalog No.:BCX0213
CAS No.:68399-17-7
- Atalafoline
Catalog No.:BCX0214
CAS No.:59-49-4
- 3'-Hydroxyliquiritin
Catalog No.:BCX0215
CAS No.:1442113-42-9
- 25,26,27-Trinor-3α-hydroxycycloartan-24-oic acid
Catalog No.:BCX0216
CAS No.:1300747-31-2
- Cyclo(L-Leu-L-Pro)
Catalog No.:BCX0217
CAS No.:2873-36-1
- Demethylmangostanin
Catalog No.:BCX0218
CAS No.:2289591-37-1
- Cyclo(L-Pro-L-Val)
Catalog No.:BCX0219
CAS No.:2854-40-2
- Cyclo(L-Pro-L-Ile)
Catalog No.:BCX0220
CAS No.:57089-60-8
A reliable validated high-performance liquid chromatography-photodiode array detection method for quantification of terpenes in Copaifera pubiflora, Copaifera trapezifolia, and Copaifera langsdorffii oleoresins.[Pubmed:36008872]
Nat Prod Res. 2024 Jan-Feb;38(2):341-346.
(The) Copaifera oleoresins are widely used in folk medicine to treat various diseases. The goal of this study was to develop a validated reverse-phase high-performance liquid chromatography method with photodiode array detection (RP-HPLC-PDA) to quantify eight terpenes: ent-hardwickiic acid, ent-Copalic acid, ent-7alpha-acetoxy hardwickiic acid, ent-16-hydroxy-3,13-clerodadiene-15,18-dioic acid, ent-5,13-labdadiene-15-oic acid, junenol, ent-kaurenoic acid, and 13E-ent-labda-7,13-dien-15-oic acid in the oleoresins of Copaifera pubiflora L. (OCP), Copaifera trapezifolia L. (OCT) and Copaifera langsdorffii L. (OCL). The linearity of the method was confirmed in the range of 20.00-500 microg.mL(-1) (r(2) > 0.999). The limit of quantification was between 1,05 and 16.89 microg.mL(-1). Precision and accuracy ranges were found to be %RSD <0.2 and 96% to 110%, respectively. Based on the obtained results, the developed analytical method is rapid, precise, accurate, and sensitive for quantifying these terpenes in Copaifera's oleoresins.
Fungal biocatalysts for labdane diterpene hydroxylation.[Pubmed:32020446]
Bioprocess Biosyst Eng. 2020 Jun;43(6):1051-1059.
Labdane diterpenes and their derivatives have shown remarkable biological activities and are useful as chiral building blocks for the synthesis of a variety of bioactive compounds. There is great interest in developing biocatalyst technology to achieve regio- and stereoselective hydroxylation of unactivated C-H bonds in complex natural products, since the functionalization of unactivated C-H bonds generally requires hard reaction conditions and highly reactive oxidizing agents, which are limited regarding the control of regio- and stereoselectivity. Filamentous fungi are efficient biocatalysts capable of catalyzing a wide variety of hydroxylation reactions, and the use of whole cell biocatalysts provides advantages regarding cofactor regeneration and is much less expensive. Therefore, the goal of this study was to select biocatalysts to develop biotransformation processes that can be scalable under mild reaction conditions for hydroxylation of a labdane diterpene, 3beta-acetoxy-Copalic acid, which contains the trans-decalin moiety and a side chain dienic system appropriate for the preparation of a variety of compounds. Biotransformation processes were carried out and five filamentous fungi were selected as capable of producing hydroxylated diterpenes at positions C-3, C-6, C-7 and C-18 of the trans-decalin moiety and C-13 of the side chain dienic system. Hydroxylation reactions occurred with regio- and stereoselectivity by using some fungi that produced only the 6alpha, 7alpha and 13alpha-hydroxyl derivatives. The chemical structures of the hydroxylated diterpenes were determined from spectrometric and spectroscopic data, and the relative stereochemistry of stereogenic centers was established from coupling constants, by NOE-diff experiments and/or by computational calculations.
Occurrence, chemical composition, biological activities and analytical methods on Copaifera genus-A review.[Pubmed:30396065]
Biomed Pharmacother. 2019 Jan;109:1-20.
Copaifera is a genus of large trees found in Brazil, mainly in Amazon forest, but also in Atlantic forest and cerrado biomes. It has also been found in other countries in South America. In Africa, it is found mainly in Congo, Cameroon, Guinea and Angola. Its oleoresin has been used in folk medicine in the treatment of numerous healthy disorders, such as urinary, respiratory, skin and inflammatory diseases, for which there are several studies corroborating its ethnopharmacological uses. It is also extensively employed in the pharmaceutical and cosmetic industries in the development of ointments, pills, soaps, perfumes, among others. Copaifera oleoresin contains mainly diterpenes, such as: kaurenoic acid, kaurenol, Copalic acid, agathic acid, hardwiickic acid, polyalthic acid, and sesquiterpenes, comprising beta-caryophyllene, caryophyllene oxide, alpha-copaene, alpha-humulene, gamma-muurolene and beta-bisabolol, among other compounds. On the other hand, Copaifera leaves contain mainly phenolic compounds, such as flavonoids and methylated galloylquinic acid derivatives. Therefore, considering the economic importance of Copaifera oleoresin, its ethnopharmacological uses, the need to develop new pharmaceuticals for the treatment of many diseases, as well as the pharmacological potential of the compounds found in Copaifera spp., it was undertaken a review covering mostly the last two decades on the distribution, chemistry, pharmacology, quality control and safety of Copaifera species.
Electrospray ionization tandem mass spectrometry of labdane-type acid diterpenes.[Pubmed:30120805]
J Mass Spectrom. 2018 Nov;53(11):1086-1096.
Copaifera (Leguminoseae) species produce a commercially interesting oleoresin that displays several biological activities, including antimicrobial and anti-inflammatory properties. Labdane-type diterpenes are the main chemical constituents of these oleoresins, and Copalic acid is the only compound that has been detected in all Copaifera oleoresins. In this study, we investigate some aspects of the gas-phase fragmentation reactions involved in the formation of the product ions from the deprotonated compounds (-)-ent-Copalic acid (1), (-)-ent-3beta-hydroxy-Copalic acid (2), (-)-ent-3beta-acetoxy-Copalic acid (3), and (-)-ent-agathic acid (4) by electrospray ionization tandem mass spectrometry (ESI-MS/MS) and multiple stage mass spectrometry (MS(n) ). Our results reveal that the product ion with m/z 99 is common to all the analyzed compounds, whereas the product ion with m/z 217 is diagnostic for compounds 2 and 3. Moreover, only compound 4 undergoes CO(2) (44 u) and acetic acid (60 u) elimination from the precursor ion. Thermochemical data obtained by computational chemistry at the B3LYP/6-31G(d) level of theory support the proposed ion structures. These data helped us to identify these compounds in a crude commercial Copaifera langsdorffii oleoresin by selective multiple reaction monitoring (MRM). Finally, a precursor ion scan (PIS) strategy aided screening of labdane-type acid diterpenes other than 1 to 4 in the same Copaifera oleoresin sample and led us to propose the structures of 8,17-dihydro-ent-agathic acid (5) and 3-keto-ent-Copalic acid (6), which have not been previously reported in Copaifera oleoresins.
ent-Copalic acid antibacterial and anti-biofilm properties against Actinomyces naeslundii and Peptostreptococcus anaerobius.[Pubmed:29885640]
Anaerobe. 2018 Aug;52:43-49.
Diterpenes are an important class of plant metabolites that can be used in the search for new antibacterial agents. ent-Copalic acid (CA), the major diterpene in Copaifera species exudates, displays several pharmacological properties. This study evaluates the CA antibacterial potential against the anaerobic bacteria Peptostreptococcus anaerobius and Actinomyces naeslundii. Antimicrobial assays included time-kill and biofilm inhibition and eradication assays. Time-kill assays conducted for CA concentrations between 6.25 and 12.5 mug/mL evidenced bactericidal activity within 72 h. CA combined with chlorhexidine dihydrochloride (CHD) exhibited bactericidal action against P. anaerobius within 6 h of incubation. As for A. naeslundii, the same combination reduced the number of microorganisms by over 3 log10 at 24 h and exerted a bactericidal effect at 48 h of incubation. CA at 500 and 2000 mug/mL inhibited P. anaerobius and A. naeslundii biofilm formation by at least 50%, respectively. CA at 62.5 and 1.000 mug/mL eradicated 99.9% of pre-formed P. anaerobius and A. naeslundii biofilms, respectively. These results indicated that CA presents in vitro antibacterial activity and is a potential biofilm inhibitory agent. This diterpene may play an important role in the search for novel sources of agents that can act against anaerobic bacteria.
Copaifera of the Neotropics: A Review of the Phytochemistry and Pharmacology.[Pubmed:29783680]
Int J Mol Sci. 2018 May 18;19(5):1511.
The oleoresin of Copaifera trees has been widely used as a traditional medicine in Neotropical regions for thousands of years and remains a popular treatment for a variety of ailments. The copaiba resins are generally composed of a volatile oil made up largely of sesquiterpene hydrocarbons, such as beta-caryophyllene, alpha-copaene, beta-elemene, alpha-humulene, and germacrene D. In addition, the oleoresin is also made up of several biologically active diterpene acids, including Copalic acid, kaurenoic acid, alepterolic acid, and polyalthic acid. This review presents a summary of the ecology and distribution of Copaifera species, the traditional uses, the biological activities, and the phytochemistry of copaiba oleoresins. In addition, several biomolecular targets relevant to the bioactivities have been implicated by molecular docking methods.
Antigenotoxicity properties of Copaifera multijuga oleoresin and its chemical marker, the diterpene (-)-copalic acid.[Pubmed:29286884]
J Toxicol Environ Health A. 2018;81(5):116-129.
In view of the biological activities and growing therapeutic interest in oleoresin obtained from Copaifera multijuga, this study aimed to determine the genotoxic and antigenotoxic potential of this oleoresin (CMO) and its chemical marker, diterpene (-)-Copalic acid (CA). The micronucleus (MN) assay in V79 cell cultures and the Ames test were used for in vitro analyses, as well as MN and comet assays in Swiss mice for in vivo analyses. The in vitro genotoxicity/mutagenicity results showed that either CMO (30, 60, or 120 microg/ml-MN assay; 0.39-3.12 mg/plate-Ames test) or CA (2.42; 4.84, or 9.7 microg/ml-MN assay; 0.39-3.12 mg/plate-Ames test) did not induce a significant effect on the frequency of MN and number of revertants, demonstrating an absence of genotoxic and mutagenic activities, respectively, in vitro. In contrast, these natural products significantly reduced the frequency of MN induced by methyl methanesulfonate (MMS), and exerted a marked inhibitory effect against indirect-acting mutagens in the Ames test. In the in vivo test system, animals treated with CMO (6.25 mg/kg b.w.) exhibited a significant decrease in rate of MN occurrence compared to those treated only with MMS. An antigenotoxic effect of CA was noted in the MN test (1 and 2 mg/kg b.w.) and the comet assay (0.5 mg/kg b.w.). Data suggest that the chemical marker of the genus Copaifera, CA, may partially be responsible for the observed chemopreventive effect attributed to CMO exposure. ABBREVIATIONS: 2-AA, 2-anthramine; 2-AF, 2-aminofluorene; AFB(1), aflatoxin B(1); B[a]P, benzo[a]pyrene; BOD, biological oxygen demand; BPDE, benzo[a]pyrene-7,8-diol-9,10-epoxide; CA, (-)-Copalic acid; CMO, oleoresin of Copaifera multijuga, DMEM, Dulbecco;s Modified Eagles;s Medium; DMSO, dimethylsulfoxide; EMBRAPA, Brazilian agricultural research corporation; GC-MS, gas chromatography-mass spectrometry; HAM-F10, nutrient mixture F-10 Ham; HPLC, high performance liquid chromatography; LC-MS, liquid chromatography-mass spectrometry; MI, mutagenic index; MMC, mitomycin C; MMS, methyl methanesulfonate; MN, micronucleus; MNPCE, micronucleated polychromatic erythrocyte; NCE, normochromatic erythrocyte; NDI, nuclear division index; NMR, nuclear magnetic resonance; NPD, 4-nitro-o-phenylenediamine; PBS, phosphate-buffered saline; PCE, polychromatic erythrocyte; SA, sodium azide; V79, Chinese hamster lung fibroblast.
Development of a Gas Chromatography Method for the Analysis of Copaiba Oil.[Pubmed:28977501]
J Chromatogr Sci. 2017 Nov 1;55(10):969-978.
A rapid, simple, precise and economic method for the quantification of main compounds of copaiba resin and essential oils (Copaifera langsdorffii Desf.) by gas chromatography (GC) has been developed and validated. Copaiba essential oil was extracted by hydrodistillation from the copaiba resin. Resin derivatization allowed the identification of diterpenes compounds. A gas chromatography-mass spectroscopy (GC/MS) method was developed to identify compounds composing the copaiba resin and essential oil. Then the GC/MS method was transposed to be used with a flame ionization detector (FID) and validated as a quantitative method. A good correlation between GC/MS and GC/FID was obtained favoring method transposition. The method showed satisfactory sensitivity, specificity, linearity, precision, accuracy, limit of detection and limit of quantitation for beta-caryophyllene, alpha-humulene and caryophyllene oxide analyses in copaiba resin and essential oils. The main compounds identified in copaiba essential oil were beta-bisabolene (23.6%), beta-caryophyllene (21.7%) and alpha-bergamotene (20.5%). Copalic acid methyl ester (15.6%), beta-bisabolene (12.3%), beta-caryophyllene (7.9%), alpha-bergamotene (7.1%) and labd-8(20)-ene-15,18-dioic acid methyl ester (6.7%) were diterpenes identified from the derivatized copaiba resin. The proposed method is suitable for a reliable separation, identification and quantification of compounds present in copaiba resin and essential oil. It could be proposed as an analytical method for the analysis of copaiba oil fraction in raw and essential oil parent extracts and after they have been incorporate in pharmaceutical formulations.
Development of a validated ultra-high-performance liquid chromatography tandem mass spectrometry method for determination of acid diterpenes in Copaifera oleoresins.[Pubmed:28789798]
J Chromatogr A. 2017 Sep 15;1515:81-90.
Species of Copaifera genus (Fabaceae - Caesalpinoiodidaeae) produces an important commercial oleoresin that displays many medicinal properties. Copaifera oleoresins (COR) are composed mainly of a mixture of diterpenes and sequiterpenes, and the main reported acid diterpenes for this genus are kaurenoic, copalic, hardwickiic and polyaltic acids. An ultra-performance liquid chromatography tandem mass spectrometry (UPLC-MS/MS) method was developed and validated for identification and quantification of nine acid diterpenes. The developed method was applied in the analyses of 10 authentic COR samples collected in the North and Southeast of Brazil and six commercial COR samples. Samples preparation consisted of simple dilution of oleoresins in methanol followed by filtration. Validation parameters of the method for nine acid diterpenes were satisfactory: selectivity/specificity was defined by retention time and MS/MS analyses for each analyte; generally all analytical curves presented r(2)>0.99, Lack-of-fit test not significant and RSD<20% for all concentration levels; limit of detection and limit of quantification were on the scale of nanogram per milliliter; inter- and intra-day precision and accuracy were adequate. Regarding the robustness, the method was sensible to small deliberate variations of temperature and additives to the mobile phase, such as formic acid and ammonium hydroxide. Results of 16 analyzed samples of COR showed qualitative and quantitative differences of acid diterpenes among all samples. The diterpenes ent-kaurenoic acid 1, ent-polyalthic acid 3, ent-Copalic acid 5 and, ent-3-beta-acetoxy Copalic acid 9 were found with more frequency in COR analyzed samples. Additionally, the content of the acid diterpenes found in 16 Copaifera oleoresin samples was analyzed by Principal Component Analysis (PCA), suggesting a botanical origin for the commercial samples. The developed UPLC method was shown to be reliable for the analysis of acid diterpenes in commercial Copaifera oleoresins.
Copaifera multijuga oleoresin and its constituent diterpene (-)-copalic acid: Genotoxicity and chemoprevention study.[Pubmed:28622827]
Mutat Res Genet Toxicol Environ Mutagen. 2017 Jul;819:26-30.
Copaiba oleoresins are used in alternative medicine as anti-inflammatory, antitumoral, and antimicrobial treatments. (-)-Copalic acid (CA) is the major diterpene found in exudates from Copaifera species. We have examined the genotoxicity and the chemopreventive potential of Copaifera multijuga oleoresin (CM) and CA. Genotoxicity assessment was examined with the peripheral blood micronucleus test and the comet assay (male Swiss mouse hepatocytes). In the chemoprevention study, we evaluated the effects of CM and CA on the formation of 1,2-dimethylhydrazine (DMH)-induced aberrant crypt foci (ACF) in male Wistar rat colon. Neither agent caused a significant increase in micronucleus frequency relative to controls, but the highest CM dose tested (400mg/kg b.w.) caused DNA damage in the comet assay. Both agents significantly reduced the frequency of DMH-induced ACF. Both CM and CA suppressed ACF formation and may have a protective effect against colon carcinogenesis.
Copalic acid analogs down-regulate androgen receptor and inhibit small chaperone protein.[Pubmed:28442254]
Bioorg Med Chem Lett. 2017 Jun 1;27(11):2292-2295.
Copalic acid, one of the diterpenoid acids in copaiba oil, inhibited the chaperone function of alpha-crystallin and heat shock protein 27kD (HSP27). It also showed potent activity in decreasing an HSP27 client protein, androgen receptor (AR), which makes it useful in prostate cancer treatment or prevention. To develop potent drug candidates to decrease the AR level in prostate cancer cells, more Copalic acid analogs were synthesized. Using the level of AR as the readout, 15 of the Copalic acid analogs were screened and two compounds were much more potent than Copalic acid. The compounds also dose-dependently inhibited AR positive prostate cancer cell growth. Furthermore, they inhibited the chaperone activity of alpha-crystallin as well.
The Unexplored Anticaries Potential of Shiitake Mushroom.[Pubmed:28082791]
Pharmacogn Rev. 2016 Jul-Dec;10(20):100-104.
Keeping an eye the escalating costs of dental services, the treatment cost of the consequences of dental caries can be reduced to manageable proportions by preventive measures aimed at decreasing the prevalence. One such measure is by increasing the consumption of caries preventive foods. Recently, there has been an upsurge of interest in mushrooms not only as a healthy food but also as a caries preventive food. The most common type of mushroom, Lentinula edodes also called as shiitake, is studied in-depth for its oral health benefits. The cultivation of shiitake dates way back to 1100 A.D. during the rule of Sung dynasty which is replaced by more modern and efficient sawdust substrate log cultures lately. Shiitake mushroom extract can be isolated in various forms such as freeze dried, oil, and ethyl acetate extracts. Various biologically active compounds such as erythritol, Copalic acid, adenosine, carvacrol, and many more are responsible for this mushroom's antimicrobial activity. Anticariogenicity can be attributed to the induction of the detachment of cariogenic microorganisms from hydroxyapatite, changes in cell surface hydrophobicity, bactericidal activity, and disruption of signal transduction in Streptococcus mutans as proved through various in vivo and in vitro studies. Apart from these benefits, it has tremendous potential to be used as an antioxidant, anticancer, antigingivitis, antifungal, and antiviral agent. The one and only known adverse reaction due to shiitake mushroom consumption is the eruption of pruritic erythematous papules termed as shiitake dermatitis. This review highlights the unexplored anticaries potential of one such useful bioactive metabolite-shiitake mushroom.
Copaifera reticulata oleoresin: Chemical characterization and antibacterial properties against oral pathogens.[Pubmed:27118478]
Anaerobe. 2016 Aug;40:18-27.
Oral infections such as periodontitis and tooth decay are the most common diseases of humankind. Oleoresins from different copaifera species display antimicrobial and anti-inflammatory activities. Copaifera reticulata is the commonest tree of this genus and grows abundantly in several Brazilian states, such as Para, Amazonas, and Ceara. The present study has evaluated the chemical composition and antimicrobial potential of the Copaifera reticulata oleoresin (CRO) against the causative agents of tooth decay and periodontitis and has assessed the CRO cytotoxic potential. Cutting edge analytical techniques (GC-MS and LC-MS) aided the chemical characterization of CRO. Antimicrobial assays included determination of the Minimum Inhibitory Concentration (MIC), determination of the Minimum Bactericidal Concentration (MBC), determination of the Minimum Inhibitory Concentration of Biofilm (MICB50), Time Kill Assay, and Checkerboard Dilution. Conduction of XTT assays on human lung fibroblasts (GM07492-A cells) helped to examine the CRO cytotoxic potential. Chromatographic analyses revealed that the major constituents of CRO were beta-bisabolene, trans-alpha-bergamotene, beta-selinene, alpha-selinene, and the terpene acids ent-agathic-15-methyl ester, ent-Copalic acid, and ent-polyalthic acid. MIC and MBC results ranged from 6.25 to 200 mug/mL against the tested bacteria. The time-kill assay conducted with CRO at concentrations between 50 and 100 mug/mL showed bactericidal activity against Fusobacterium nucleatum (ATCC 25586) and Streptococcus mitis (ATCC 49456) after 4 h, Prevotella nigrescens (ATCC 33563) after 6 h, Porphyromonas gingivalis (ATCC 33277) and Lactobacillus casei (clinical isolate) after 12 h, and Streptococcus salivarius (ATCC 25975) and Streptococcus mutans (ATCC 25175) after 18 h. The fractional inhibitory concentration indexes (FICIs) revealed antagonistic interaction for Lactobacillus casei (clinical isolate), indifferent effect for Porphyromonas gingivalis (ATCC 33277), Fusobacterium nucleatum (ATCC 25586), Prevotella nigrescens (ATCC 33563), and Streptococcus salivarius (ATCC 25975), and additive effect for Streptococcus mutans (ATCC 25175) and Streptococcus mitis (ATCC 49456). Treatment of GM07492-A cells with CRO demonstrated that concentrations up to 39 mug/mL significantly reduced cell viability as compared to the negative control, being IC50 equal to 51.85 +/- 5.4 mug/mL. These results indicated that CRO plays an important part in the search for novel sources of agents that can act against oral pathogens.


