CrinineCAS# 510-67-8 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

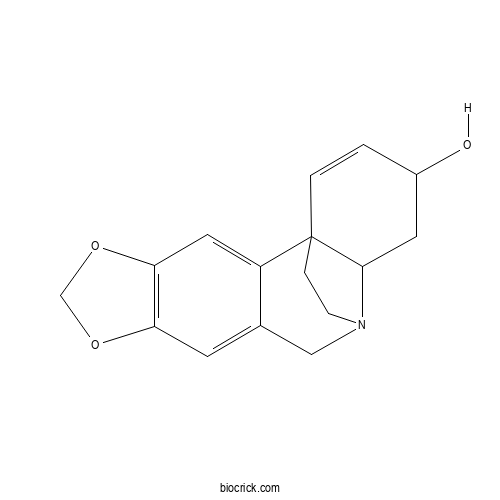
| Cas No. | 510-67-8 | SDF | Download SDF |
| PubChem ID | 101727 | Appearance | Powder |
| Formula | C16H17NO3 | M.Wt | 271.31 |
| Type of Compound | Alkaloids | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | 5,7-dioxa-12-azapentacyclo[10.5.2.01,13.02,10.04,8]nonadeca-2,4(8),9,16-tetraen-15-ol | ||
| SMILES | C1CN2CC3=CC4=C(C=C3C15C2CC(C=C5)O)OCO4 | ||
| Standard InChIKey | RPAORVSEYNOMBR-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C16H17NO3/c18-11-1-2-16-3-4-17(15(16)6-11)8-10-5-13-14(7-12(10)16)20-9-19-13/h1-2,5,7,11,15,18H,3-4,6,8-9H2 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Crinine Dilution Calculator

Crinine Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.6858 mL | 18.4291 mL | 36.8582 mL | 73.7164 mL | 92.1455 mL |
| 5 mM | 0.7372 mL | 3.6858 mL | 7.3716 mL | 14.7433 mL | 18.4291 mL |
| 10 mM | 0.3686 mL | 1.8429 mL | 3.6858 mL | 7.3716 mL | 9.2146 mL |
| 50 mM | 0.0737 mL | 0.3686 mL | 0.7372 mL | 1.4743 mL | 1.8429 mL |
| 100 mM | 0.0369 mL | 0.1843 mL | 0.3686 mL | 0.7372 mL | 0.9215 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- 10-Hydroxyoleuropein
Catalog No.:BCX0202
CAS No.:84638-44-8
- Denticulatain E
Catalog No.:BCX0201
CAS No.:1919050-76-2
- Gnetuhainin N
Catalog No.:BCX0200
CAS No.:337464-99-0
- 4α-Hydroxybisabola-2,10-diene-1,9-dione
Catalog No.:BCX0199
CAS No.:1627567-79-6
- Heliobuphthalmin lactone
Catalog No.:BCX0198
CAS No.:580-73-4
- Macowine
Catalog No.:BCX0197
CAS No.:307494-75-3
- Methyl pechueloate
Catalog No.:BCX0196
CAS No.:83161-59-5
- Gelse-norursane B
Catalog No.:BCX0195
CAS No.:2072868-77-8
- Hippadine
Catalog No.:BCX0194
CAS No.:52886-06-3
- Neoolivil
Catalog No.:BCX0193
CAS No.:1277182-38-3
- 2β,15α-Dihydroxy-ent-kaur-16-ene
Catalog No.:BCX0192
CAS No.:34302-36-8
- Petiolin G
Catalog No.:BCX0191
CAS No.:1204251-11-5
- Kolavenic acid
Catalog No.:BCX0204
CAS No.:25436-90-2
- Clauszoline B
Catalog No.:BCX0205
CAS No.:185508-03-6
- 14,15-Dinorcleroda-3,11E-dien-13-one
Catalog No.:BCX0206
CAS No.:1186523-96-5
- Diplacone
Catalog No.:BCX0207
CAS No.:73676-38-7
- Copalic acid
Catalog No.:BCX0208
CAS No.:24470-48-2
- 1-Epideacetylbowdensine
Catalog No.:BCX0209
CAS No.:101219-55-0
- Fibraurin
Catalog No.:BCX0210
CAS No.:25254-84-6
- Pratorimine
Catalog No.:BCX0211
CAS No.:88660-12-2
- Gnetuhainin O
Catalog No.:BCX0212
CAS No.:337464-95-6
- (2R,3S)-Pterosin C
Catalog No.:BCX0213
CAS No.:68399-17-7
- Atalafoline
Catalog No.:BCX0214
CAS No.:59-49-4
- 3'-Hydroxyliquiritin
Catalog No.:BCX0215
CAS No.:1442113-42-9
Chiral Bisphosphine-Catalyzed Asymmetric Staudinger/aza-Wittig Reaction: An Enantioselective Desymmetrizing Approach to Crinine-Type Amaryllidaceae Alkaloids.[Pubmed:38642063]
J Am Chem Soc. 2024 Apr 20.
An unprecedented chiral bisphosphine-catalyzed asymmetric Staudinger/aza-Wittig reaction of 2,2-disubstituted cyclohexane-1,3-diones is reported, enabling the facile access of a broad range of cis-3a-arylhydroindoles in high yields with excellent enantioselectivities. The key to the success of this work relies on the first application of chiral bisphosphine DuanPhos to the asymmetric Staudinger/aza-Wittig reaction. An effective reductive system has been established to address the challenging P(V) horizontal lineO/P(III) redox cycle associated with the chiral bisphosphine catalyst. In addition, comprehensive experimental and computational investigations were carried out to elucidate the mechanism of the asymmetric reaction. Leveraging the newly developed chemistry, the enantioselective total syntheses of several Crinine-type Amaryllidaceae alkaloids, including (+)-powelline, (+)-buphanamine, (+)-vittatine, and (+)-crinane, have been accomplished with remarkable conciseness and efficiency.
A mini-review of the anti-SARS-CoV-2 potency of Amaryllidaceae alkaloids.[Pubmed:38579643]
Phytomedicine. 2024 Mar 29;129:155576.
BACKGROUND: Nature has perennially served as an infinite reservoir of diverse chemicals with numerous applications benefiting humankind. In recent years, due to the emerging COVID-19 pandemic, there has been a surge in studies on repurposing natural products as anti-SARS-CoV-2 agents, including plant-derived substances. Among all types of natural products, alkaloids remain one of the most important groups with various known medicinal values. The current investigation focuses on Amaryllidaceae alkaloids (AAs) since AAs have drawn significant scientific attention as anti-SARS-CoV-2 agents over the past few years. PURPOSE AND STUDY DESIGN: This study serves as a mini-review, summarizing recent advances in studying the anti-SARS-CoV-2 potency of AAs, covering two aspects: structure-activity relationship and mechanism of action (MOA). METHODS: The study covers the period from 2019 to 2023. The information in this review were retrieved from common databases including Web of Science, ScienceDirect, PubMed and Google scholar. Reported anti-SARS-CoV-2 potency, cytotoxicity and possible biological targets of AAs were summarized and classified into different skeletal subclasses. Then, the structure-activity relationship (SAR) was explored, pinpointing the key pharmacophore-related structural moieties. To study the mechanism of action of anti-SARS-CoV-2 AAs, possible biological targets were discussed. RESULTS: In total, fourteen research articles about anti-SARS-CoV-2 was selected. From the SAR point of view, four skeletal subclasses of AAs (lycorine-, galanthamine-, Crinine- and homolycorine-types) appear to be promising for further investigation as anti-SARS-CoV-2 agents despite experimental inconsistencies in determining in vitro half maximal inhibitory effective concentration (EC(50)). Narciclasine, haemanthamine- and montanine-type skeletons were cytotoxic and devoid of anti-SARS-CoV-2 activity. The lycorine-type scaffold was the most structurally diverse in this study and preliminary structure-activity relationships revealed the crucial role of ring C and substituents on rings A, C and D in its anti-SARS-CoV-2 activity. It also appears that two enantiomeric skeletons (haemanthamine- and Crinine-types) displayed opposite activity/toxicity profiles regarding anti-SARS-CoV-2 activity. Pharmacophore-related moieties of the haemanthamine/Crinine-type skeletons were the substituents on rings B, C and the dioxymethylene moiety. All galanthamine-type alkaloids in this study were devoid of cytotoxicity and it appears that varying substituents on rings C and D could enhance the anti-SARS-CoV-2 potency. Regarding MOAs, initial experimental results suggested Mpro and RdRp as possible viral targets. Dual functionality between anti-inflammatory activity on host cells and anti-SARS-CoV-2 activity on the SARS-CoV-2 virus of isoquinoline alkaloids, including AAs, were suggested as the possible MOAs to alleviate severe complications in COVID-19 patients. This dual functionality was proposed to be related to the p38 MAPK signaling pathway. CONCLUSION: Overall, Amaryllidaceae alkaloids appear to be promising for further investigation as anti-SARS-CoV-2 agents. The skeletal subclasses holding the premise for further investigation are lycorine-, Crinine-, galanthamine- and homolycorine-types.
Molecular Docking and GC/MS-Based Approach for Identification of Anxiolytic Alkaloids from Griffinia (Amaryllidaceae) Species in a Zebrafish Model.[Pubmed:38354224]
Chem Biodivers. 2024 Mar;21(3):e202302122.
Griffinia gardneriana Ravenna, Griffinia liboniana Morren and Griffinia nocturna Ravenna (Amarillydaceae) are bulbous plants found in tropical regions of Brazil. Our work aimed to determine the alkaloid profiles of Griffinia spp. and evaluate their anxiolytic potential through in vivo and in silico assays. The plants grown in greenhouses were dried and their ground bulbs were subjected to liquid-liquid partitions, resulting in alkaloid fractions that were analyzed by gas chromatography coupled to mass spectrometry (GC-MS). Anxiolytic activity was evaluated in zebrafish (Danio rerio) through intraperitoneal injection at doses of 40, 100 and 200 mg/kg in light-dark box test. GC-MS analyses revealed 23 alkaloids belonging to different skeleton types: lycorine, homolychorine, galanthamine, Crinine, haemanthamine, montanine and narcisclasine. The chemical profiles were relatively similar, presenting 8 alkaloids common to the three species. The major component for G. gardneriana and G. liboniana was lycorine, while G. nocturna consisted mainly of anhydrolycorine. All three alkaloid fractions demonstrated anxiolytic effect. Furthermore, pre-treatment with diazepam and pizotifen drugs was able to reverse the anxiolytic action, indicating involving the GABAergic and serotonergic receptors. Molecular docking showed that the compounds vittatine, lycorine and 11,12-dehydro-2-methoxyassoanine had high affinity with both receptors, suggesting them to be responsible for the anxiolytic effect.
Antileishmanial Activity of Clinanthus milagroanthus S. Leiva & Meerow (Amaryllidaceae) Collected in Peru.[Pubmed:36679035]
Plants (Basel). 2023 Jan 10;12(2):322.
Leishmaniasis is a worldwide infectious parasitic disease caused by different species of protozoa of the genus Leishmania, which are transmitted to animals and humans through the bite of insects of the Psychodidae family. In the present work, the antileishmanial activity of an alkaloid extract of the bulbs of Clinanthus milagroanthus S. Leiva & Meerow (Amaryllidaceae) was evaluated in vitro, in vivo, and in silico against the parasite Leishmania braziliensis, and the chemical profile of the sample was determined by GC-MS analysis. At concentrations of 1, 10, and 100 microg.mL-1, the alkaloid extract presented inhibition percentages of 8.7%, 23.1%, and 98.8%, respectively, against L. braziliensis with a p < 0.05, and IC50 values of 18.5 +/- 0.3 microg.mL-1. Furthermore, at a dose of 1.0 mg.kg-1, a greater decrease in lesion size was observed (90%) for in vivo assays, as well as a decrease in infection (96%), finding no significant differences (p > 0.05) in comparison with amphotericin B (92% and 98%, respectively). Eleven alkaloids were identified in C. milagroanthus bulbs: galanthamine, vittatine/Crinine, 8-O-demethylmaritidine, anhydrolycorine, 11,12-dehydroanhydrolycorine, hippamine, lycorine, 2-hydroxyanhydrolycorine, 7-hydroxyclivonine, 2alpha-hydroxyhomolycorine, and 7-hydroxyclivonine isomer. A molecular model of Leishmania braziliensis trypanothione reductase (TRLb) was built using computational experiments to evaluate in silico the potential of the Amaryllidaceae alkaloid identified in C. milagroanthus toward this enzyme. The structures galanthamine, 7-hydroxyclivonine isomer, and Crinine showed better estimated free energy of binding than the reference compound, amphotericin B. In conclusion, this is the first in vitro, in vivo, and in silico report about the antileishmanial potential and alkaloid profiling of the extract of C. milagroanthus bulbs, which could become an interesting source of bioactive molecules.
Epimeric Mixture Analysis and Absolute Configuration Determination Using an Integrated Spectroscopic and Computational Approach-A Case Study of Two Epimers of 6-Hydroxyhippeastidine.[Pubmed:36615407]
Molecules. 2022 Dec 26;28(1):214.
Structural elucidation has always been challenging, and misassignment remains a stringent issue in the field of natural products. The growing interest in discovering unknown, complex natural structures accompanies the increasing awareness concerning misassignments in the community. The combination of various spectroscopic methods with molecular modeling has gained popularity in recent years. In this work, we demonstrated, for the first time, its power to fully elucidate the 2-dimensional and 3-dimensional structures of two epimers in an epimeric mixture of 6-hydroxyhippeastidine. DFT calculation of chemical shifts was first performed to assist the assignment of planar structures. Furthermore, relative and absolute configurations were established by three different ways of computer-assisted structure elucidation (CASE) coupled with ORD/ECD/VCD spectroscopies. In addition, the significant added value of OR/ORD computations to relative and absolute configuration determination was also revealed. Remarkably, the differentiation of two enantiomeric scaffolds (Crinine and haemanthamine) was accomplished via OR/ORD calculations with cross-validation by ECD and VCD.
Structurally diverse alkaloids with nine frameworks from Zephyranthes candida and their acetylcholinesterase inhibitory and anti-inflammatory activities.[Pubmed:36535411]
Phytochemistry. 2023 Mar;207:113564.
Twenty-six structurally diverse Amaryllidaceae alkaloids, including ten undescribed compounds named zephyranines A-I and 6-O-ethylnerinine, two undescribed natural products zephyranthine-6-one and 3-O-deacetyl-sternbergine, were isolated from whole plants of Zephyranthes candida. Their structures were determined by HRESIMS, 1D and 2D NMR, CD data analysis, NMR and ECD calculations, and single-crystal X-ray diffraction analysis. All structures were classified into nine framework types: 10b,11-seco-Crinine, graciline, Crinine, homolycorine, trisphaeridine, lycorine, galasine, tazettine, and belladine. Zephyranine A represents the first naturally occurring 10b,11-seco-Crinine type alkaloid, and zephyranine B is the sixth graciline type alkaloid. 6-O-ethylnerinine is an artifact from the extraction and isolation. All isolates were evaluated for their acetylcholinesterase (AChE) inhibitory and anti-inflammatory activities. Zephyranines A, G, and H exhibited moderate AChE inhibitory activities, with IC(50) values of 8.2, 39.0, and 10.8 muM, respectively. Zephyranine B, haemanthamine, haemanthidine, 11-hydroxyvittatine, and 8-demethoxy-10-O-methylhostasine exhibited potent anti-inflammatory activity on the LPS-induced NO production in RAW264.7 mouse macrophages with IC(50) values of 21.3, 4.6, 12.2, 5.6, and 17.4 muM, respectively. Structure-activity-relationship analysis and docking studies indicated that interactions with the key Trp286 and Tyr337 residues are required for potent AChE inhibitors.
A General Electro-Synthesis Approach to Amaryllidaceae Alkaloids.[Pubmed:35662286]
Chemistry. 2022 Sep 6;28(50):e202201523.
Amaryllidaceae alkaloids appeal to organic chemists with their attractive structures and their impressive antitumor and acetylcholinesterase inhibitory properties. We demonstrate a highly versatile access to this family of natural products. A general protocol with high yields in a sustainable electro-organic key transformation on a metal-free anode to spirodienones facilitates functionalization to the alkaloids. The biomimetic syntheses start with the readily available, inexpensive biogenic starting materials methyl gallate, O-methyl tyramine, and vanillin derivatives. Through known dynamic resolutions, this technology provides access to both enantiomeric series of (epi-)martidine, (epi-)Crinine, siculine, and galantamine, clinically prescribed for the treatment of Alzheimer's disease.
Lycorine Alkaloid and Crinum americanum L. (Amaryllidaceae) Extracts Display Antifungal Activity on Clinically Relevant Candida Species.[Pubmed:35566325]
Molecules. 2022 May 6;27(9):2976.
Candida species are the main fungal agents causing infectious conditions in hospital patients. The development of new drugs with antifungal potential, increased efficacy, and reduced toxicity is essential to face the challenge of fungal resistance to standard treatments. The aim of this study is to evaluate the in vitro antifungal effects of two crude extracts of Crinum americanum L., a rich alkaloid fraction and lycorine alkaloid, on the Candida species. As such, we used a disk diffusion susceptibility test, determined the minimum inhibitory concentration (MIC), and characterized the components of the extracts using Electrospray Ionization Fourier Transform Ion Cyclotron Resonance Mass Spectrometry (ESI FT-ICR MS). The extracts were found to have antifungal activity against various Candida species. The chemical characterization of the extracts indicated the presence of alkaloids such as lycorine and Crinine. The Amaryllidaceae family has a promising antifungal potential. Furthermore, it was found that the alkaloid lycorine directly contributes to the effects that were observed for the extracts and fraction of C. americanum.
In vitro and in silico analysis of galanthine from Zephyranthes carinata as an inhibitor of acetylcholinesterase.[Pubmed:35483192]
Biomed Pharmacother. 2022 Jun;150:113016.
Zephyranthes carinata Herb., a specie of the Amaryllidoideae subfamily, has been reported to have inhibitory activity against acetylcholinesterase. However, scientific evidence related to their bioactive alkaloids has been lacking. Thus, this study describes the isolation of the alkaloids of this plant, and their inhibition of the enzymes acetylcholinesterase (eeAChE) and butyrylcholinesterase (eqBuChE), being galanthine the main component. Additionally, haemanthamine, hamayne, lycoramine, lycorine, tazettine, trisphaeridine and vittatine/Crinine were also isolated. The results showed that galanthine has significant activity at low micromolar concentrations for eeAChE (IC(50) = 1.96 mug/mL). The in-silico study allowed to establish at a molecular level the high affinity and the way galanthine interacts with the active site of the TcAChE enzyme, information that corroborates the result of the experimental IC(50). However, according to molecular dynamics (MD) analysis, it is also suggested that galanthine presents a different inhibition mode that the one observed for galanthamine, by presenting interaction with peripheral anionic binding site of the enzyme, which prevents the entrance and exit of molecules from the active site. Thus, in vitro screening assays plus rapid computer development play an essential role in the search for new cholinesterase inhibitors by identifying unknown bio-interactions between bioactive compounds and biological targets.
Assessment on facile Diels-Alder approach of alpha-pyrone and terpenoquinone for the expedient synthesis of various natural scaffolds.[Pubmed:35357593]
Nat Prod Bioprospect. 2022 Mar 31;12(1):12.
The development of highly facile synthetic procedures for the expedient synthesis of complex natural molecules is always in demand. As this aspect, the Diels-Alder reaction (DAR) has a versatile approach to the synthesis of complex natural compounds and highly regio-/stereoselcetive heterocyclic scaffolds. Additionally, alpha-pyrone and terpenoquinone are two versatile key intermediates that are prevalent in various bioactive natural compounds for instance, (+/-)-Crinine, (+/-)-joubertinamine, (+/-)-pancratistatin, (-)-cyclozonarone, and 8-ephipuupehedione, etc. Hence, the current review summarizes the Diels-Alder reaction application of alpha-pyrone and terpenoquinone to the constructive synthesis of various natural products over the past two decades (2001-2021). Equally, it serves as a stencil for the invention and development of new synthetic strategies for high-complex molecular structured natural and heterocyclic molecules.
Amaryllidaceae Alkaloid Cherylline Inhibits the Replication of Dengue and Zika Viruses.[Pubmed:34152811]
Antimicrob Agents Chemother. 2021 Aug 17;65(9):e0039821.
Dengue fever, caused by dengue virus (DENV), is the most prevalent arthropod-borne viral disease and is endemic in many tropical and subtropical parts of the world, with an increasing incidence in temperate regions. The closely related flavivirus Zika virus (ZIKV) can be transmitted vertically in utero and causes congenital Zika syndrome and other birth defects. In adults, ZIKV is associated with Guillain-Barre syndrome. There are no approved antiviral therapies against either virus. Effective antiviral compounds are urgently needed. Amaryllidaceae alkaloids (AAs) are a specific class of nitrogen-containing compounds produced by plants of the Amaryllidaceae family with numerous biological activities. Recently, the AA lycorine was shown to present strong antiflaviviral properties. Previously, we demonstrated that Crinum jagus contained lycorine and several alkaloids of the cherylline, Crinine, and galanthamine types with unknown antiviral potential. In this study, we explored their biological activities. We show that C. jagus crude alkaloid extract inhibited DENV infection. Among the purified AAs, cherylline efficiently inhibited both DENV (50% effective concentration [EC(50)], 8.8 muM) and ZIKV replication (EC(50), 20.3 muM) but had no effect on HIV-1 infection. Time-of-drug-addition and -removal experiments identified a postentry step as the one targeted by cherylline. Consistently, using subgenomic replicons and replication-defective genomes, we demonstrate that cherylline specifically hinders the viral RNA synthesis step but not viral translation. In conclusion, AAs are an underestimated source of antiflavivirus compounds, including the effective inhibitor cherylline, which could be optimized for new therapeutic approaches.
The Chemical Synthesis of the Crinine and Haemanthamine Alkaloids: Biologically Active and Enantiomerically-Related Systems that Serve as Vehicles for Showcasing New Methodologies for Molecular Assembly.[Pubmed:33540725]
Molecules. 2021 Feb 2;26(3):765.
The title alkaloids, often referred to collectively as Crinines, are a prominent group of structurally distinct natural products with additional members being reported on a regular basis. As such, and because of their often notable biological properties, they have attracted attention as synthetic targets since the mid-1950s. Such efforts continue unabated and more recent studies on these alkaloids have focused on using them as vehicles for showcasing the utility of new synthetic methods. This review provides a comprehensive survey of the nearly seventy-year history of these synthetic endeavors.
Alkaloids of Phaedranassa dubia (Kunth) J.F. Macbr. and Phaedranassa brevifolia Meerow (Amaryllidaceae) from Ecuador and its cholinesterase-inhibitory activity.[Pubmed:32982003]
S Afr J Bot. 2021 Jan;136:91-99.
Alzheimer's disease is considered the most common cause of dementia and, in an increasingly aging population worldwide, the quest for treatment is a priority. Amaryllidaceae alkaloids are of main interest because of their cholinesterase inhibition potential, which is the main palliative treatment available for this disease. We evaluated the alkaloidal profile and the in vitro inhibitory activity on acetylcholinesterase (AChE) and butyrylcholinesterase (BuChE) of bulb alkaloid extract of Phaedranassa dubia and Phaedranassa brevifolia collected in Ecuador. Using gas chromatography coupled to mass spectrometry (GC-MS), we identified typical Amaryllidaceae alkaloids in these species, highlighting the presence of lycorine-type alkaloids in P. dubia and haemanthamine/Crinine-type in P. brevifolia. The species P. dubia and P. brevifolia showed inhibitory activities against AChE (IC(50) values of 25.48 +/- 0.39 and 3.45 +/- 0.29 mug.mL(-1), respectively) and BuChE (IC(50) values of 114.96 +/- 4.94 and 58.89 +/- 0.55 mug.mL(-1), respectively). Computational experiments allowed us to understand the interactions of the alkaloids identified in these samples toward the active sites of AChE and BuChE. In silico, some alkaloids detected in these Amaryllidaceae species presented higher estimated binding free energy toward BuChE than galanthamine. This is the first study about the alkaloid profile and biological potential of P. brevifolia species.
Stereoselective, Multicomponent Approach to Quaternary Substituted Hydroindole Scaffolds.[Pubmed:32610919]
Org Lett. 2020 Jul 2;22(13):5079-5084.
The Amaryllidaceae alkaloids have been a target of synthesis for decades due to their complex architectures and biological activity. A central feature of these natural product cores is a quaternary substituted hydroindole heterocycle. Building off the foundation of our previous multicomponent approach to highly functionalized pyrrolidinones, herein we report a highly convergent, diastereoselective, multicomponent approach to access the hydroindole cores present within Crinine, haemanthamine, pretazettine, and various other bioactive alkaloids. These scaffolds are additionally useful as building blocks for druglike molecules and natural product like library generation.
Gigantelline, gigantellinine and gigancrinine, cherylline- and crinine-type alkaloids isolated from Crinum jagus with anti-acetylcholinesterase activity.[Pubmed:32335411]
Phytochemistry. 2020 Jul;175:112390.
Three undescribed Amarylidaceae alkaloids, named gigantelline, gigantellinine and giganCrinine, were isolated from Crinum jagus (syn. = Crinum giganteum) collected in Senegal, together with the already known sanguinine, cherylline, lycorine, Crinine, flexinine and the isoquinolinone derivative hippadine. Gigantelline, gigantellinine and giganCrinine were characterized as 4-(6,7-dimethoxy-2-methyl-1,2,3,4-tetrahydro-isoquinolin-4-yl)-phenol, its 7-O-demethyl-5ꞌ-hydroxy-4ꞌ-methoxy derivative and 5,6a,7,7a,8a,9-hexahydro-6,9a-ethano[1,3]dioxolo[4,5-j]oxireno[2,3-b]phenanthridin-9-ol, respectively, by using spectroscopic (1D and 2D (1)H and (13)C NMR and HRESIMS) and chemical methods. Their relative configuration was assigned by NOESY NMR spectra and NMR calculations, while the absolute configuration was assigned using electronic circular dichroism (ECD) experiments and calculations. Sanguinine, cherylline, Crinine, flexinine, and the isoquinolinone hippadine, were isolated for the first time from C. jagus. Cherylline, gigantellinine, Crinine, flexinine and sanguinine inhibited the activity of AChE in a dose-dependent manner, and inhibition by sanguinine was remarkably effective (IC(50) = 1.83 +/- 0.01 muM). Cherylline and hippadine showed weak cytotoxicity at 100 muM.


