DiplaconeCAS# 73676-38-7 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 73676-38-7 | SDF | Download SDF |
| PubChem ID | 639465 | Appearance | Powder |
| Formula | C25H28O6 | M.Wt | 424.5 |
| Type of Compound | Flavonoids | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
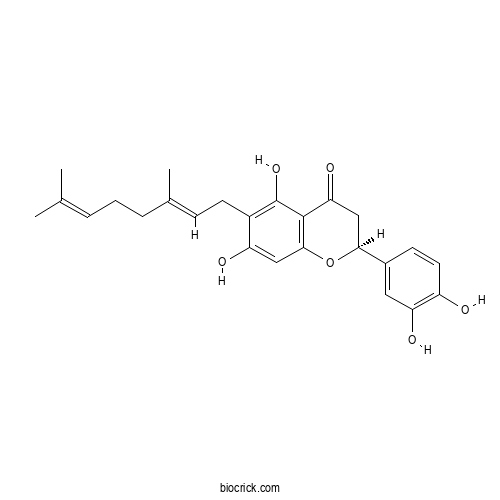
| Chemical Name | (2S)-2-(3,4-dihydroxyphenyl)-6-[(2E)-3,7-dimethylocta-2,6-dienyl]-5,7-dihydroxy-2,3-dihydrochromen-4-one | ||
| SMILES | CC(=CCCC(=CCC1=C(C2=C(C=C1O)OC(CC2=O)C3=CC(=C(C=C3)O)O)O)C)C | ||
| Standard InChIKey | XCYSQFHYFNWYFP-CEMXSPGASA-N | ||
| Standard InChI | InChI=1S/C25H28O6/c1-14(2)5-4-6-15(3)7-9-17-19(27)12-23-24(25(17)30)21(29)13-22(31-23)16-8-10-18(26)20(28)11-16/h5,7-8,10-12,22,26-28,30H,4,6,9,13H2,1-3H3/b15-7+/t22-/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Diplacone Dilution Calculator

Diplacone Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.3557 mL | 11.7786 mL | 23.5571 mL | 47.1143 mL | 58.8928 mL |
| 5 mM | 0.4711 mL | 2.3557 mL | 4.7114 mL | 9.4229 mL | 11.7786 mL |
| 10 mM | 0.2356 mL | 1.1779 mL | 2.3557 mL | 4.7114 mL | 5.8893 mL |
| 50 mM | 0.0471 mL | 0.2356 mL | 0.4711 mL | 0.9423 mL | 1.1779 mL |
| 100 mM | 0.0236 mL | 0.1178 mL | 0.2356 mL | 0.4711 mL | 0.5889 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- 14,15-Dinorcleroda-3,11E-dien-13-one
Catalog No.:BCX0206
CAS No.:1186523-96-5
- Clauszoline B
Catalog No.:BCX0205
CAS No.:185508-03-6
- Kolavenic acid
Catalog No.:BCX0204
CAS No.:25436-90-2
- Crinine
Catalog No.:BCX0203
CAS No.:510-67-8
- 10-Hydroxyoleuropein
Catalog No.:BCX0202
CAS No.:84638-44-8
- Denticulatain E
Catalog No.:BCX0201
CAS No.:1919050-76-2
- Gnetuhainin N
Catalog No.:BCX0200
CAS No.:337464-99-0
- 4α-Hydroxybisabola-2,10-diene-1,9-dione
Catalog No.:BCX0199
CAS No.:1627567-79-6
- Heliobuphthalmin lactone
Catalog No.:BCX0198
CAS No.:580-73-4
- Macowine
Catalog No.:BCX0197
CAS No.:307494-75-3
- Methyl pechueloate
Catalog No.:BCX0196
CAS No.:83161-59-5
- Gelse-norursane B
Catalog No.:BCX0195
CAS No.:2072868-77-8
- Copalic acid
Catalog No.:BCX0208
CAS No.:24470-48-2
- 1-Epideacetylbowdensine
Catalog No.:BCX0209
CAS No.:101219-55-0
- Fibraurin
Catalog No.:BCX0210
CAS No.:25254-84-6
- Pratorimine
Catalog No.:BCX0211
CAS No.:88660-12-2
- Gnetuhainin O
Catalog No.:BCX0212
CAS No.:337464-95-6
- (2R,3S)-Pterosin C
Catalog No.:BCX0213
CAS No.:68399-17-7
- Atalafoline
Catalog No.:BCX0214
CAS No.:59-49-4
- 3'-Hydroxyliquiritin
Catalog No.:BCX0215
CAS No.:1442113-42-9
- 25,26,27-Trinor-3α-hydroxycycloartan-24-oic acid
Catalog No.:BCX0216
CAS No.:1300747-31-2
- Cyclo(L-Leu-L-Pro)
Catalog No.:BCX0217
CAS No.:2873-36-1
- Demethylmangostanin
Catalog No.:BCX0218
CAS No.:2289591-37-1
- Cyclo(L-Pro-L-Val)
Catalog No.:BCX0219
CAS No.:2854-40-2
Perspectives on antimicrobial properties of Paulownia tomentosa Steud. fruit products in the control of Staphylococcus aureus infections.[Pubmed:37979817]
J Ethnopharmacol. 2024 Mar 1;321:117461.
ETHNOPHARMACOLOGICAL RELEVANCE: Paulownia tomentosa Steud. (P. tomentosa) is a medium-sized tree traditionally used in Chinese folk medicine for the treatment of infectious diseases. It is a rich source of prenylated phenolic compounds that have been extensively studied for their promising biological activities. AIM OF THE STUDY: Due to the increasing development of antibiotic resistance, our study investigated plant-derived natural products from the fruits of P. tomentosa that could control Staphylococcus aureus infections with novel targets/modes of action and reduce antimicrobial resistance. MATERIALS AND METHODS: The ethanolic extract was fractionated and detected by liquid chromatography. The antistaphylococcal effects of the plant formulations were studied in detail in vitro by various biological methods, including microdilution methods for minimum inhibitory concentration (MIC), the checkerboard titration technique for synergy assay, fluorescence measurements for membrane disruption experiments, autoinducer-2-mediated bioassay for quorum sensing inhibition, and counting of colony-forming units for relative adhesion. Morphology was examined by transmission electron microscopy. RESULTS: Total ethanolic extract and chloroform fraction showed MICs of 128 and 32 mug/mL, respectively. Diplacol, Diplacone, and 3'-O-methyl-5'-hydroxyDiplacone inhibited S. aureus growth in the range of 8-16 mug/mL. Synergistic potential was shown in combination with mupirocin and fusidic acid. The ethanolic extract and the chloroform fraction destroyed the cell membranes by 91.61% and 79.46%, respectively, while the pure compounds were less active. The ethanolic extract and the pure compounds reduced the number of adhered cells to 47.33-10.26% compared to the untreated control. All tested plant formulations, except Diplacone, inhibited quorum sensing of S. aureus. Transmission electron microscopy showed deformation of S. aureus cells. CONCLUSIONS: The products from the fruit of P. tomentosa showed antimicrobial properties against S. aureus alone and in combination with antibiotics. By affecting intracellular targets, geranylated flavonoids proposed novel approaches in the control of staphylococcal infections.
Diplacone Isolated from Paulownia tomentosa Mature Fruit Induces Ferroptosis-Mediated Cell Death through Mitochondrial Ca(2+) Influx and Mitochondrial Permeability Transition.[Pubmed:37108220]
Int J Mol Sci. 2023 Apr 11;24(8):7057.
The recently defined type of cell death ferroptosis has garnered significant attention as a potential new approach to cancer treatment owing to its more immunogenic nature when compared with apoptosis. Ferroptosis is characterized by the depletion of glutathione (GSH)/glutathione peroxidase-4 (GPx4) and iron-dependent lipid peroxidation. Diplacone (DP), a geranylated flavonoid compound found in Paulownia tomentosa fruit, has been identified to have anti-inflammatory and anti-radical activity. In this study, the potential anticancer activity of DP was explored against A549 human lung cancer cells. It was found that DP induced a form of cytotoxicity distinct from apoptosis, which was accompanied by extensive mitochondrial-derived cytoplasmic vacuoles. DP was also shown to increase mitochondrial Ca(2+) influx, reactive oxygen species (ROS) production, and mitochondrial permeability transition (MPT) pore-opening. These changes led to decreases in mitochondrial membrane potential and DP-induced cell death. DP also induced lipid peroxidation and ATF3 expression, which are hallmarks of ferroptosis. The ferroptosis inhibitors ferrostatin-1 and liproxstatin-1 were effective in counteracting the DP-mediated ferroptosis-related features. Our results could contribute to the use of DP as a ferroptosis-inducing agent, enabling studies focusing on the relationship between ferroptosis and the immunogenic cell death of cancer cells.
Prenylated Flavonoids in Topical Infections and Wound Healing.[Pubmed:35889363]
Molecules. 2022 Jul 13;27(14):4491.
The review presents prenylated flavonoids as potential therapeutic agents for the treatment of topical skin infections and wounds, as they can restore the balance in the wound microenvironment. A thorough two-stage search of scientific papers published between 2000 and 2022 was conducted, with independent assessment of results by two reviewers. The main criteria were an MIC (minimum inhibitory concentration) of up to 32 microg/mL, a microdilution/macrodilution broth method according to CLSI (Clinical and Laboratory Standards Institute) or EUCAST (European Committee on Antimicrobial Susceptibility Testing), pathogens responsible for skin infections, and additional antioxidant, anti-inflammatory, and low cytotoxic effects. A total of 127 structurally diverse flavonoids showed promising antimicrobial activity against pathogens affecting wound healing, predominantly Staphylococcus aureus strains, but only artocarpin, Diplacone, isobavachalcone, licochalcone A, sophoraflavanone G, and xanthohumol showed multiple activity, including antimicrobial, antioxidant, and anti-inflammatory along with low cytotoxicity important for wound healing. Although prenylated flavonoids appear to be promising in wound therapy of humans, and also animals, their activity was measured only in vitro and in vivo. Future studies are, therefore, needed to establish rational dosing according to MIC and MBC (minimum bactericidal concentration) values, test potential toxicity to human cells, measure healing kinetics, and consider formulation in smart drug release systems and/or delivery technologies to increase their bioavailability.
Direct and Indirect Antioxidant Effects of Selected Plant Phenolics in Cell-Based Assays.[Pubmed:33926137]
Molecules. 2021 Apr 26;26(9):2534.
Background: Oxidative stress is a key factor in the pathophysiology of many diseases. This study aimed to verify the antioxidant activity of selected plant phenolics in cell-based assays and determine their direct or indirect effects. Methods: The cellular antioxidant assay (CAA) assay was employed for direct scavenging assays. In the indirect approach, the influence of each test substance on the gene and protein expression and activity of selected antioxidant enzymes was observed. One assay also dealt with activation of the Nrf2-ARE pathway. The overall effect of each compound was measured using a glucose oxidative stress protection assay. Results: Among the test compounds, acteoside showed the highest direct scavenging activity and no effect on the expression of antioxidant enzymes. It increased only the activity of catalase. Diplacone was less active in direct antioxidant assays but positively affected enzyme expression and catalase activity. Morusin showed no antioxidant activity in the CAA assay. Similarly, pomiferin had only mild antioxidant activity and proved rather cytotoxic. Conclusions: Of the four selected phenolics, only acteoside and Diplacone demonstrated antioxidant effects in cell-based assays.
Antiproliferative and cytotoxic activities of C-Geranylated flavonoids from Paulownia tomentosa Steud. Fruit.[Pubmed:33901796]
Bioorg Chem. 2021 Jun;111:104797.
Prenylated or geranylated flavonoids have been studied for their promising antiproliferative and cytotoxic activities. Twelve natural geranylated flavonoids (1-12) were isolated from the fruit of Paulownia tomentosa Steud. Their structures were elucidated using UV and IR spectroscopy, mass spectrometry, and 1D and 2D NMR spectroscopy. The absolute configurations were determined using NMR and circular dichroism. Seven of the compounds were characterized as new geranylated derivatives isolated from a natural source for the first time, namely 3'-O-methyl-5'-hydroxyisoDiplacone (3), pauloDiplacone A (5), tomentone II (6), tomentone B (7), tomentoDiplacone P (8), pauloDiplacone B (9), and tomentoflavone A (12). After 24 h of incubation at concentrations in the range 1-30 muM, the isolated compounds were tested for their antiproliferative and cytotoxic potentials against the human monocytic leukaemia cell line THP-1, using WST-1 and LDH assays, respectively. Almost all of the test compounds induced a concentration-dependent reduction in the metabolic activity of THP-1 cells and a concentration-dependent reduction in the cell viability. Diplacone (1) was the most potent antiproliferative and cytotoxic agent (IC(50) 9.31 +/- 0.72 muM, LC(50) 18.01 +/- 1.19 microM). 3'-O-Methyl-5'-hydroxyDiplacone (2) showed relatively strong antiproliferative effect (IC(50) 12.61 +/- 0.90 muM) and weaker cytotoxic activity (LC(50) > 30 muM), indicating that it may serve as a potential lead compound for further testing. The structure-activity relationship for the 12 isolated compounds is discussed.
Anti-inflammatory potential of composites of yeast glucan particles and geranylated flavonoid diplacone.[Pubmed:32972156]
Ceska Slov Farm. 2020 Summer;69(3):130-136.
Geranylated flavanone Diplacone is a flavanone iso- lated from Paulownia tomentosa (Thunb.) Steud. (Paulowniaceae) with anti-inflammatory and antioxidant properties, nevertheless showing high lipophilicity and low solubility in water. Diplacone was therefore used as a model molecule for incorporation into glucan particles (GPs). GPs are prepared by intensive washing of yeast (Saccharomyces cerevisiae) leading to hollow shells consisting of β-(13)/β-(16) glucan mainly. The aim of this study was to compare anti-inflammatory potential of GPs-Diplacone composites with the compound itself, GPs themselves and the physical mixture of GPs and Diplacone. The cell line THP1-XBlueTM-MD2-CD14 derived from human leukemic monocytes was stimulated with lipopolysaccharide (LPS) from Escherichia coli to trigger inflammatory reaction. The composites of GPs with Diplacone significantly decreased the activity of pro-inflammatory transcription factors nuclear factor κB (NF-κB) and activator protein 1 (AP-1).
Glucan particles as suitable carriers for the natural anti-inflammatory compounds curcumin and diplacone - Evaluation in an ex vivo model.[Pubmed:32320720]
Int J Pharm. 2020 May 30;582:119318.
Natural compounds offer a wide spectrum of potential active substances, but often they have a poor bioavailability. To increase the bioavailability and bioactivity of the natural anti-inflammatory molecules curcumin and Diplacone, we used glucan particles (GPs), hollow shells from Saccharomyces cerevisiae composed mainly of beta-1,3-d-glucan. Their indigestibility and relative stability in the gut combined with their immunomodulatory effects makes them promising carriers for such compounds. This study aimed to determine how curcumin and Diplacone, either alone or incorporated in GPs, affect the immunomodulatory activity of the latter by assessing the respiratory burst response and the secretion of pro-inflammatory cytokines by primary porcine innate immune cells. Incorporating curcumin and Diplacone into GPs by controlled evaporation of the organic solvent substantially reduced the respiratory burst response mediated by GPs. Incorporated curcumin in GPs also reduced GPs mediated secretion of IL-1beta and TNF-alpha by innate immune cells. The obtained results indicate a potentially beneficial effect of the incorporation of curcumin or Diplacone into GPs against inflammation.
Novel cannabis flavonoid, cannflavin A displays both a hormetic and neuroprotective profile against amyloid beta-mediated neurotoxicity in PC12 cells: Comparison with geranylated flavonoids, mimulone and diplacone.[Pubmed:31437460]
Biochem Pharmacol. 2019 Nov;169:113609.
BACKGROUND: Flavonoids form a diverse class of naturally occurring polyphenols ascribed various biological activities, including inhibition of amyloid beta (Abeta) fibrillisation and neurotoxicity of relevance to Alzheimer's disease. Cannabis contains a unique subset of prenylated flavonoids, the cannflavins. While selected conventional flavonoids have demonstrated anti-amyloid and neuroprotective potential, any neuroprotective bioactivity of prenylated flavonoids has not been determined. We evaluated the in vitro neuroprotective and anti-aggregative properties of the novel geranylated cannabis-derived flavonoid, cannflavin A against Abeta(1-42) and compared it to two similarly geranylated flavonoids, mimulone and Diplacone, to compare the bioactive properties of these unique flavonoids more broadly. METHODS: Neuronal viability were assessed in PC12 cells biochemically using the MTT assay in the presence of each flavonoid (1-200 microM) for 48 h. Sub-toxic threshold test concentrations of each flavonoid were then applied to cells, alone or with concomitant incubation with the lipid peroxidant tert-butyl hyrdroperoxide (t-bhp) or amyloid beta (Abeta(1-42); 0-2 microM). Fluorescent staining was used to indicate effects of Abeta(1-42) on PC12 cellular morphology, while direct effects of each flavonoid on Abeta fibril formation and aggregation were assessed using the Thioflavin T (ThT) fluorometric kinetic assay and transmission electron microscopy (TEM) to visualise fibril and aggregate morphology. RESULTS: Cannflavin A demonstrated intrinsic hormetic effects on cell viability, increasing viability by 40% from 1 to 10 microM but displaying neurotoxicity at higher (>10-100 microM) concentrations. Neither mimulone nor Diplacone exhibited such a biphasic effect, instead showing only concentration-dependent neurotoxicity, with Diplacone the more potent (from >1 microM). However at the lower concentrations (<10 microM), cannflavin A increased cell viability by up to 40%, while 10 microM cannflavin A inhibited the neurotoxicity elicited by Abeta(1-42) (0-2 microM), reducing Abeta aggregate adherence to PC-12 cells and associated neurite loss. The neuroprotective effects of cannflavin A were associated with a direct inhibition of Abeta(1-42) fibril and aggregate density, evidenced by attenuated ThT fluorescence kinetics and microscopic evidence of both altered and diminished density of Abeta aggregate and fibril morphology via electron microscopy. CONCLUSIONS: These findings highlight a concentration-dependent hormetic and neuroprotective role of cannflavin A against Abeta-mediated neurotoxicity, associated with an inhibition of Abeta fibrillisation. The efficacy of the cannabis flavone may itself direct further lead development targeting neurodegeneration in Alzheimer's disease. However, the geranylated flavonoids generally displayed a comparatively potent neurotoxicity not observed with many conventional flavonoids in vitro.
Paulownia C-geranylated flavonoids: their structural variety, biological activity and application prospects.[Pubmed:32214921]
Phytochem Rev. 2019;18(3):549-570.
Paulownia species, especially their flowers and fruits, are traditionally used in Chinese herbal medicines for the treatment of infectious diseases. C-geranylated flavonoids were found to be the major special metabolites in Paulownia flowers and fruits, and 76 C-geranylated flavonoids had been isolated and characterized thus far. Structural variations in Paulownia C-geranylated flavonoids are mainly due to the complicated structural modifications in their geranyl substituents. These natural compounds have attracted much attention because of their various biological activities, including antioxidation, anti-inflammation, cytotoxic activity and various enzymatic inhibitions, etc. Among them, Diplacone, a major Paulownia component, was considered to have promise for applications in medicine. This paper summarizes the information from current publications on Paulownia C-geranylated flavonoids, with a focus on their structural variety, key spectroscopic characteristics, biological activity with structure-activity relationships and application prospects. We hope that this paper will stimulate further investigations of Paulownia species and this kind of natural product.
Identification of C-geranylated flavonoids from Paulownia catalpifolia Gong Tong fruits by HPLC-DAD-ESI-MS/MS and their anti-aging effects on 2BS cells induced by H(2)O(2).[Pubmed:28558874]
Chin J Nat Med. 2017 May;15(5):384-391.
The fruits of Paulownia catalpifolia Gong Tong are used as a Chinese folk herbal medicine for the treatment of enteritis, tonsillitis, bronchitis, and dysentery, etc. Our previous study has identified new C-geranylated flavanones with obvious anti-proliferative effects in lung cancer A549 cells. In the present study, a new C-geranylated flavone, paucatalinone C (1) and five known C-geranylated flavanones (2-6) were isolated. In addition, a total of 34 C-geranylated flavonoids were detected by HPLC-DAD-ESI-MS/MS coupling techniques from the CH(2)Cl(2) extract of P. catalpifolia. Futhermore, anti-aging effects of isolated compounds were evaluated in vitro with premature senescent 2BS cells induced by H(2)O(2). Phytochemical results indicated that P. catalpifolia was a natural resource of abundant C-geranylated flavonoids. Diplacone (3) and paucatalinone A (5) were the potent anti-aging agents in the premature senescent 2BS cells induced by H(2)O(2) and the C-geranyl substituent may be an important factor because of its lipophilic character.
Anti-inflammatory Activity of Natural Geranylated Flavonoids: Cyclooxygenase and Lipoxygenase Inhibitory Properties and Proteomic Analysis.[Pubmed:28322565]
J Nat Prod. 2017 Apr 28;80(4):999-1006.
Geranyl flavones have been studied as compounds that potentially can be developed as anti-inflammatory agents. A series of natural geranylated flavanones was isolated from Paulownia tomentosa fruits, and these compounds were studied for their anti-inflammatory activity and possible mechanism of action. Two new compounds were characterized [paulownione C (17) and tomentoDiplacone O (20)], and all of the isolated derivatives were assayed for their ability to inhibit cyclooxygenases (COX-1 and COX-2) and 5-lipoxygenase (5-LOX). The compounds tested showed variable degrees of activity, with several of them showing activity comparable to or greater than the standards used in COX-1, COX-2, and 5-LOX assays. However, only the compound tomentoDiplacone O (20) showed more selectivity against COX-2 versus COX-1 when compared with ibuprofen. The ability of the test compounds to interact with the above-mentioned enzymes was supported by docking studies, which revealed the possible incorporation of selected test substances into the active sites of these enzymes. Furthermore, one of the COX/LOX dual inhibitors, Diplacone (14) (a major geranylated flavanone of P. tomentosa), was studied in vitro to obtain a proteomic overview of its effect on inflammation in LPS-treated THP-1 macrophages, supporting its previously observed anti-inflammatory activity and revealing the mechanism of its anti-inflammatory effect.
Protective activity of C-geranylflavonoid analogs from Paulownia tomentosa against DNA damage in 137Cs irradiated AHH-1 cells.[Pubmed:25918796]
Nat Prod Commun. 2014 Sep;9(9):1295-8.
Radiotherapy is an important form of treatment for a wide range of cancers, but it can damage DNA and cause adverse effects. We investigated if the Diplacone analogs of P. tomentosa were radio-protective in a human lymphoblastoid cell line (AHH-1). Four geranylated flavonoids, Diplacone, 3'-O-methyl-5'-hydroxyDiplacone, 3'-O-methyl-5'-O-methylDiplacone and 3'-O-methyldiplacol, were tested for their antioxidant and radio-protective effects. Diplacone analogs effectively scavenged free radicals and inhibited radiation-induced DNA strand breaks in vitro. They significantly decreased levels of reactive oxygen species and cellular DNA damage in 2 Gy-irradiated AHH-1 cells. Glutathione levels and superoxide dismutase activity in irradiated AHH-1 cells increased significantly after treatment with these analogs. The enhanced biological anti-oxidant activity and radioprotective activity of Diplacone analogs maintained the survival of irradiated AHH-1 cells in a clonogenic assay. These data suggest that Diplacone analogs may protect healthy tissue surrounding tumor cells during radiotherapy to ensure better control of radiotherapy and allow higher doses of radiotherapy to be employed.
Diplacone and mimulone ameliorate dextran sulfate sodium-induced colitis in rats.[Pubmed:25623260]
Fitoterapia. 2015 Mar;101:201-7.
Diplacone (1) and mimulone (2), two geranylated flavanones, have previously shown anti-inflammatory and antiradical activity in vitro. The present study aimed to evaluate their activity in vivo on a model of colitis induced in Wistar rats by an oral administration of dextran sulfate sodium (DSS). Diplacone (1) and mimulone (2) were administered at a bolus dose of 25mg/kg by gastric gavage 48 and 24h prior to the induction of colitis by DSS and every 24h on the following days of the experiment. The effect of the treatment was assessed by monitoring the disease activity index (DAI), histopathological examination, evaluation of the weight and length of the colon and by analysis of the levels and activities of cyclooxygenase-2 (COX-2), matrix metalloproteinase-2 (MMP2), superoxide dismutase-2 (SOD2), and catalase (CAT) in the inflamed tissue. Administration of the test compounds prior and after induction of colitis ameliorated the symptoms of colitis (diarrhea, presence of the blood in the stool) and delayed their onset. The ability of compounds 1 and 2 to reduce the levels of COX-2 and to increase the ratio of pro-MMP2/MMP2 activity correlates with the values of the DAI. The lowering of the levels of the antioxidant enzymes SOD2 and CAT reflects the ability of the test compounds to scavenge reactive oxygen species.


