Kolavenic acidCAS# 25436-90-2 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 25436-90-2 | SDF | Download SDF |
| PubChem ID | 6441458 | Appearance | Oil |
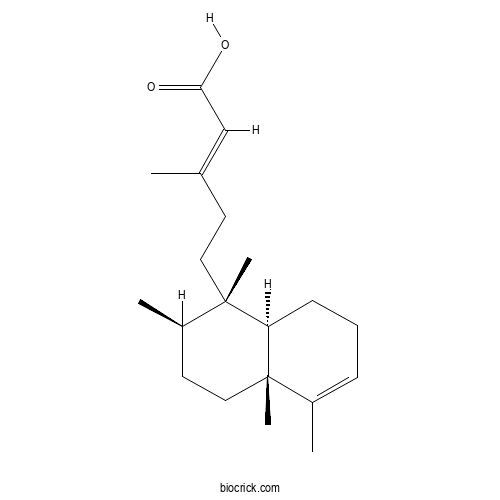
| Formula | C20H32O2 | M.Wt | 304.5 |
| Type of Compound | Diterpenoids | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | (E)-5-[(1S,2R,4aR,8aR)-1,2,4a,5-tetramethyl-2,3,4,7,8,8a-hexahydronaphthalen-1-yl]-3-methylpent-2-enoic acid | ||
| SMILES | CC1CCC2(C(C1(C)CCC(=CC(=O)O)C)CCC=C2C)C | ||
| Standard InChIKey | NLVMTSRTOGOFQD-MWRQYBNOSA-N | ||
| Standard InChI | InChI=1S/C20H32O2/c1-14(13-18(21)22)9-11-19(4)16(3)10-12-20(5)15(2)7-6-8-17(19)20/h7,13,16-17H,6,8-12H2,1-5H3,(H,21,22)/b14-13+/t16-,17-,19+,20+/m1/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Kolavenic acid Dilution Calculator

Kolavenic acid Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.2841 mL | 16.4204 mL | 32.8407 mL | 65.6814 mL | 82.1018 mL |
| 5 mM | 0.6568 mL | 3.2841 mL | 6.5681 mL | 13.1363 mL | 16.4204 mL |
| 10 mM | 0.3284 mL | 1.642 mL | 3.2841 mL | 6.5681 mL | 8.2102 mL |
| 50 mM | 0.0657 mL | 0.3284 mL | 0.6568 mL | 1.3136 mL | 1.642 mL |
| 100 mM | 0.0328 mL | 0.1642 mL | 0.3284 mL | 0.6568 mL | 0.821 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Crinine
Catalog No.:BCX0203
CAS No.:510-67-8
- 10-Hydroxyoleuropein
Catalog No.:BCX0202
CAS No.:84638-44-8
- Denticulatain E
Catalog No.:BCX0201
CAS No.:1919050-76-2
- Gnetuhainin N
Catalog No.:BCX0200
CAS No.:337464-99-0
- 4α-Hydroxybisabola-2,10-diene-1,9-dione
Catalog No.:BCX0199
CAS No.:1627567-79-6
- Heliobuphthalmin lactone
Catalog No.:BCX0198
CAS No.:580-73-4
- Macowine
Catalog No.:BCX0197
CAS No.:307494-75-3
- Methyl pechueloate
Catalog No.:BCX0196
CAS No.:83161-59-5
- Gelse-norursane B
Catalog No.:BCX0195
CAS No.:2072868-77-8
- Hippadine
Catalog No.:BCX0194
CAS No.:52886-06-3
- Neoolivil
Catalog No.:BCX0193
CAS No.:1277182-38-3
- 2β,15α-Dihydroxy-ent-kaur-16-ene
Catalog No.:BCX0192
CAS No.:34302-36-8
- Clauszoline B
Catalog No.:BCX0205
CAS No.:185508-03-6
- 14,15-Dinorcleroda-3,11E-dien-13-one
Catalog No.:BCX0206
CAS No.:1186523-96-5
- Diplacone
Catalog No.:BCX0207
CAS No.:73676-38-7
- Copalic acid
Catalog No.:BCX0208
CAS No.:24470-48-2
- 1-Epideacetylbowdensine
Catalog No.:BCX0209
CAS No.:101219-55-0
- Fibraurin
Catalog No.:BCX0210
CAS No.:25254-84-6
- Pratorimine
Catalog No.:BCX0211
CAS No.:88660-12-2
- Gnetuhainin O
Catalog No.:BCX0212
CAS No.:337464-95-6
- (2R,3S)-Pterosin C
Catalog No.:BCX0213
CAS No.:68399-17-7
- Atalafoline
Catalog No.:BCX0214
CAS No.:59-49-4
- 3'-Hydroxyliquiritin
Catalog No.:BCX0215
CAS No.:1442113-42-9
- 25,26,27-Trinor-3α-hydroxycycloartan-24-oic acid
Catalog No.:BCX0216
CAS No.:1300747-31-2
Isolation of metallic salts of cytotoxic clerodanes from medicinal plant Polyalthia longifolia var. pendula.[Pubmed:36861230]
Pak J Pharm Sci. 2022 Nov;35(6(Special)):1691-1698.
Isolation of sodium and potassium salt of Kolavenic acid (1,2), as a mixture of (3:1) and sodium and potassium salt of 16 oxo-cleroda-3,13(14) E-dien-15-oic acid (3, 4) as a mixture of (1:1) are first time reported form reddish black ripe and green unripe berries of Polyalthia longifolia var. pendula respectively. Three known constituents obtained, were identified as cleroda-3, 13(14) E-dien-15-oic acid (Kolavenic acid) (5), 16(R and S)-hydroxy cleroda-3,13 (14)Z-dien-15,16-olide (6) and 16 oxo-cleroda-3,13(14) E-dien-15-oic acid (7). Structures of all these compounds have been determined through spectral studies while metal analyses were carried out to confirm the structure of the salts. Compounds 3, 4 and 7 possess cytotoxic activity against lung (NCI-H460), oral (CAL-27) and normal mouse fibroblast (NCI-3T3) cancer cell lines. Diterpenoid (7), a bioprivileged, compound shows potent cytotoxic activity against oral cancer cell line (CAL-27) with IC(50) 11.3+/-0.6microg/mL in comparison with the standard 5-flourouracil (IC(50) 12.7+/-0.1microg/mL) and lungs cancer cell lines (NCI-H460) with IC(50) 5.3+/-0.2microg/mL as compared to the standard drug cisplatin (IC(50) 5.7+/-0.2microg/mL).
KIFC1 regulates ZWINT to promote tumor progression and spheroid formation in colorectal cancer.[Pubmed:33819373]
Pathol Int. 2021 Jul;71(7):441-452.
Colorectal cancer (CRC) is the second leading cause of cancer-related mortality worldwide. Kinesin Family Member C1 (KIFC1) has been proposed as a promising therapeutic target due to its pivotal role in centrosome clustering to mediate cancer cell progression. This study aimed to analyze the expression and biological function of KIFC1 in CRC. Immunohistochemically, 67 (52%) of 129 CRC cases were positive for KIFC1 and statistically associated with poorer overall survival. KIFC1 small interfering RNA (siRNA)-transfected cells demonstrated lower cell proliferation as compared to the negative control cells. A specific KIFC1 inhibitor, Kolavenic acid analog (KAA) drastically inhibited CRC cell proliferation. Microarray analysis revealed that KAA-treated CRC cells presented reduced ZW10 interacting kinetochore protein (ZWINT) expression as compared to control cells. Immunohistochemical analysis demonstrated that 61 (47%) of 129 CRC cases were positive for ZWINT and ZWINT expression was significantly correlated with KIFC1 expression. ZWINT-positive cases exhibited significantly worse overall survival. KIFC1 siRNA-transfected cells showed reduced ZWINT expression while ZWINT siRNA-transfected cells decreased cell proliferation. Both KIFC1 and ZWINT knockdown cells attenuated spheroid formation ability. This study provides new insights into KIFC1 regulating ZWINT in CRC progression and its potential as a therapeutic target.
Antiamoebic activity of plant-based natural products and their conjugated silver nanoparticles against Acanthamoeba castellanii (ATCC 50492).[Pubmed:32016777]
AMB Express. 2020 Feb 3;10(1):24.
Acanthamoeba spp. are the causative agent of Acanthamoeba keratitis and granulomatous amoebic encephalitis (GAE). The current options to treat Acanthamoeba infections have limited success. Silver nanoparticles show antimicrobial effects and enhance the efficacy of their payload at the specific biological targets. Natural folk plants have been widely used for treating diseases as the phytochemicals from several plants have been shown to exhibit amoebicidal effects. Herein, we used natural products of plant or commercial sources including quercetin (QT), Kolavenic acid (PGEA) isolated from plant extracts of Polyalthia longifolia var pendula and crude plant methanolic extract of Caesalpinia pulcherrima (CPFLM) as antiacanthamoebic agents. Furthermore, these plant-based materials were conjugated with silver nanoparticles (AgNPs) to determine the effects of the natural compounds and their nanoconjugates against a clinical isolate of A. castellanii from a keratitis patient (ATCC 50492) belonging to the T4 genotype. The compounds were conjugated with AgNPs and characterized by using ultraviolet visible spectrophotometry and atomic force microscopy. Quercetin coated silver nanoparticles (QT-AgNPs) showed characteristic surface plasmon resonance band at 443 nm and the average size distribution was found to be around 45 nm. The natural compounds alone and their nanoconjugates were tested for the viability of amoebae, encystation and excystation activity against A. castellanii. The natural compounds showed significant growth inhibition of A. castellanii while QT-AgNPs specifically exhibited enhanced antiamoebic effects as well as interrupted the encystation and excystation activity of the amoebae. Interestingly, these compounds and nanoconjugates did not exhibit in vitro cytotoxic effects against human cells. Plant-based compounds and extracts could be an interesting strategy in development of alternative therapeutics against Acanthamoeba infections.
Kolavenic acid analog restores growth in HSET-overproducing fission yeast cells and multipolar mitosis in MDA-MB-231 human cells.[Pubmed:31753800]
Bioorg Med Chem. 2020 Jan 1;28(1):115154.
Although cancer cells often harbor supernumerary centrosomes, they form pseudo-bipolar spindles via centrosome clustering, instead of lethal multipolar spindles, and thus avoid cell death. Kinesin-14 HSET/KIFC1 is a crucial protein involved in centrosome clustering. Accordingly, a compound that targets HSET could potentially inhibit cancer cell proliferation in a targeted manner. Here, we report three natural compounds derived from Solidago altissima that restored the growth of fission yeast cells exhibiting lethal HSET overproduction (positive screening), namely solidagonic acid (SA) (1), Kolavenic acid analog (KAA: a stereo isomer at C-9 and C-10 of 6beta-tigloyloxyKolavenic acid) (2), and Kolavenic acid (KA) (3). All three compounds suppressed fission yeast cell death and enabled reversion of the mitotic spindles from a monopolar to bipolar morphology. Compound 2, which exerted the strongest activity against HSET-overproducing yeast cells, also inhibited centrosome clustering in MDA-MB-231 human breast adenocarcinoma cells, which contained large numbers of supernumerary centrosomes. These natural compounds may be useful as bioprobes in studies of HSET function. Moreover, compound 2 is a prime contender in the development of novel agents for cancer treatment.
Chemical defense against insects in Heterotheca subaxillaris and three Orobanchaceae species using exudates from trichomes.[Pubmed:30828973]
Pest Manag Sci. 2019 Sep;75(9):2474-2481.
BACKGROUND: One of the roles of plant trichomes is thought to be reducing feeding damage from herbivores. Among trichomes, glandular trichomes play a role in chemical defense systems in plants by means of stored biologically active phytochemicals. These phytochemicals act as pest repellents. They show antimicrobial and insecticidal activities, and they have also been isolated and identified from wild plants. RESULTS: The Asteraceae species Heterotheca subaxillaris has many glandular trichomes on the leaf surface, and these contain sesquiterpene carboxylates, which show insect antifeedant activity. Because these sesquiterpene carboxylates are major constituents of glandular trichomes, they may act as a chemical defense in H. subaxillaris. The Orobanchaceae species Parentucellia viscosa also has many glandular trichomes on the leaf surface and produces an insect antifeedant clerodane-type diterpene, Kolavenic acid, in these trichomes. Additionally, two other Orobanchaceae species, Bellardia trixago and Parentucellia latifolia, also have many glandular trichomes, but the constituents of these glandular trichomes did not show biological activities against test insects. However, the seco-labdane diterpene alcohol trixagol and its hemi-malonate were major constituents in B. trixago, and these terpenes may act as physical defenses against herbivores by interfering with feeding due to their viscosity. CONCLUSION: The secondary metabolites from glandular trichomes of H. subaxillaris and P. viscosa showed insect antifeedant activity, and these secondary metabolites were presumed to act as chemical defenses for these plant species. On the other hand, non-biologically active secondary metabolites produced by two other Orobanchaceae, B. trixago and P. latifolia, were presumed to act as physical defenses due to their viscosity. Defense systems such as these may be applicable to new crop breeding to enhance protection against insect pests. (c) 2019 Society of Chemical Industry.
Quantification of diterpene acids in Copaiba oleoresin by UHPLC-ELSD and heteronuclear two-dimensional qNMR.[Pubmed:30086505]
J Pharm Biomed Anal. 2018 Oct 25;160:126-134.
In this study, we present the quantitation of eight diterpene acids in the oleoresin of Copaifera reticulata Ducke by UHPLC-ELSD and quantitative HSQC (heteronuclear single quantum correlation spectroscopy). UHPLC was performed using reversed phase material and external calibration and showed RSD values of Kolavenic acid was identified as a major diterpene acid in the oleoresin of Copaifera reticulata, with amounts of 4.0 +/- 0.3%.
Antibiofilm potential of 16-oxo-cleroda-3, 13(14) E-diene-15 oic acid and its five new gamma-amino gamma-lactone derivatives against methicillin resistant Staphylococcus aureus and Streptococcus mutans.[Pubmed:28692914]
Eur J Med Chem. 2017 Sep 29;138:480-490.
The clerodane diterpenoids 16-oxo-cleroda-3, 13(14) E-diene-15 oic acid (1) and Kolavenic acid (2) isolated from Polyalthia longifolia var. pendula (Linn.) were previously reported for their antimicrobial activity. Thus present study was designed to investigate the biofilm inhibiting potential of these diterpenoids (1-2) and five new lactone derivatives (3-7) of 1 against methicillin resistant Staphylococcus aureus (MRSA), Streptococcus mutans, Klebsiella pneumoniae and Proteus mirabilis. Compounds 1 and 3 at 10-20 mug/mL were found to be bacteriostatic and significantly reduced the biofilm formation and metabolically active cells of MRSA and S. mutans up to 90%. Parental diterpenoid (1) at 10 and 16 mug/mL significantly eradicated the preformed biofilm of both pathogens. Both the compounds also delayed acid production at minimum inhibitory (MIC) and sub-inhibitory concentrations (sub MIC). Florescence and scanning electron microscopy further confirms the biofilm inhibiting potential of compounds 1 and 3 and displayed disrupted biofilms at MIC and sub MIC levels. The gene expression of MRSA and S. mutans responsible for biofilm formation was also altered. Moreover, the observed anti-virulence properties and delayed bacterial growth after 10 min of exposure to the test compounds 1 and 3 make them a promising class of antibiofilm agents.
Physicochemical and antimicrobial properties of copaiba oil: implications on product quality control.[Pubmed:28068029]
Acta Sci Pol Technol Aliment. 2015 Jul-Sep;14(3):215-225.
BACKGROUND: The copaiba oil is a common natural product used in cosmetic industry and as a nutraceutical product. However, lack of quality control and scarce knowledge about its antimicrobial activity is a point of concern. The proposal of this study was to investigate the physicochemical properties and the antimicrobial activity of five commercial brands of copaiba oil. METHODS: Acidity and ester index, refractory index, solubility in alcohol, and thin layer chromatography were performed to verify the physicochemical properties of five commercial copaiba oils sold in local pharmacies. Ultra performance liquid chromatography coupled with diode-array detection and electrospray ionization quadrupole time-of-flight mass spectrometry (UPLC-DAD/ESI-Q-TOF-MS) was used to investigate diterpene acids while the volatile compounds were analysed by gas chromatography-mass spectrometry (GC-MS). Antibacterial and antifungal activities were also evaluated by agar diffusion technique; and minimal inhibitory concentration and maximal bactericidal concentration were defined for each sample and bacteria. RESULTS: The physical-chemical analysis revealed heterogeneity between all samples analysed. The A1 sample showed characteristics of copaiba oil and was mainly composed by hydrocarbon sesquiterpenes (29.95% beta-bisabolene, 25.65% Z-alpha-bergamotene and 10.27% beta-cariophyllene). Among diterpene acids, the UPLCDAD/ESI-Q-TOF-MS data are compatible with presence of copalic and/or Kolavenic acid (m/z 305 [M + H]+). Candida albicans was sensitive to almost all samples at high concentration and Saccaromyces. Cerevisiae showed sensitivity to A1 sample at 100 mg/mL. Although variable, all samples showed antibacterial activity. Significant activity was seen for A3 (19.0 +/-0 and 15.6 +/-0.5 mm), A4 (16.6 +/-0.5 and 15.6 +/-0 mm), and A5 (17.1 +/-0 and 17.1 +/-0 mm) on Staphylococcus saprophyticus and S. aureus, respectively. All samples were active against Klebsiella pneumoniae showing >/=15 mm diameter halo inhibition; and only A2 was active against Eschirichia coli. Phytopatogens tested revealed resistance of Ralstonia solanacearum CGH12 to all samples and susceptibility of Xcv 112 strain of Xanthomonas campestris pv campestris to almost all samples. MIC and MMC showed bacteriostatic effect against clinical interest bacteria and bactericidal effect against phytopatogens. CONCLUSIONS: The results from physicochemical analysis reinforce the fact that it is imperative to include simple conventional methods in the analysis of oil products. The analysis of copaiba oil gives safe products and purity which ensure products with quality. Also, since copaiba oil is an over-the-counter product the results indicate that pharmacosurveillance must be improved by the governmental regulation agency to avoid microorganism resistance selection and to achieve better international quality products.
Clerodane diterpenes isolated from Polyalthia longifolia induce apoptosis in human leukemia HL-60 cells.[Pubmed:24088522]
J Oleo Sci. 2013;62(10):843-8.
Polyalthia is a versatile genus of shrubs and trees found in tropic and sub-tropic regions. In this study, three clerodane diterpenes, Kolavenic acid (1), polyalthialdoic acid (2), and 16alpha-hydroxy-cleroda-3,13(14)Z-dien-15,16-olide (3) isolated from Polyalthia longifolia leaves were evaluated for their apoptotic potential against human leukemia HL-60 cells. Compounds 2 and 3 inhibited cell proliferation with IC(5)(0) values of 21.8 and 13.7 muM, respectively. Morphological changes and DNA fragmentation analysis indicated that these diterpenes induce apoptotic cell death in the HL-60 cells. Our results revealed the importance of P. longifolia as a chemopreventive medicinal plant.
An unusual bisnor-clerodane diterpenoid from Polyalthia simiarum.[Pubmed:21121244]
Nat Prod Commun. 2010 Oct;5(10):1543-6.
The stem bark of Polyalthia simiarum has yielded a new bisnor-type clerodane diterpenoid, 2-oxo-14,15-bisnor-3,11E-kolavadien-13-one (1), and three previously known clerodane derivatives, Kolavenic acid (2), 16beta-hydroxycleroda-3,13(14)Z-dien-15,16-olide (3), and 16-oxocleroda-3,13(14)E-dien-15-oic acid (4). The structures of these compounds were unambiguously determined by extensive NMR studies as well as by comparison with related compounds. Till now this is the second report of the occurrence of any unusual C-18 clerodane diterpenoid from nature. The crude light petroleum extract and the purified compound 3 demonstrated moderate free radical scavenging activity with IC50 values of 21.5 and 23.5 microg/mL, respectively.
Antimicrobial activity of various parts of Polyalthia longifolia var. pendula: isolation of active principles from the leaves and the berries.[Pubmed:18389472]
Phytother Res. 2008 Jul;22(7):907-12.
Methanol extracts of leaves, stem, twigs, green berries, flowers, roots, root-wood and root-bark of Polyalthia longifolia var. pendula, were tested for their antibacterial and antifungal potentials. Bioassay monitored isolation work on the methanol extract of leaves and berries which possess promising antibacterial activity led to the isolation of seven clerodane diterpenoids, 16(R and S)-hydroxy-cleroda-3,13(14)Z-dien-15,16-olide (1), 16-oxo-cleroda-3,13(14)E-dien-15-oic acid (2), methyl-16-oxo-cleroda-3,13(14)E-dien-15-oate (3), 2-oxo-Kolavenic acid (4), 16 (R and S)-hydroxy-cleroda-3,13(14)Z-dien-15,16-olide-2-one (5), (4-->2)-abeo-16(R and S)-hydroxy-cleroda-2,13(14)Z-dien-15,16-olide-3-al (6), 3beta,16alpha-dihydroxy-cleroda-4(18), 13(14)Z-dien-15,16-olide (7), while Kolavenic acid (8) and solidagonal acid (9) were obtained from the root-wood. Diterpenoids 1 and 8 were also obtained from the root-bark. It is the first report of the isolation of 7 and 9 from this source, and clerodane 3 was obtained as a natural product for the first time. Clerodanes 1, 2, 5, 6 and 7 were found to be active antimicrobial agents with MIC values ranging between 7.8 and 500 microg/mL. Diterpenoid 1 emerged as the most active antimicrobial agent. The acetyl derivative (10) of 1 and the methyl derivative (3) of 2 were found to be less active than the parent compounds. A complex of allantoin was also obtained from the berries, which on hydrolysis furnished pure allantoin (11).
Evaluation of insecticidal activity of diterpenes and lignans from Aristolochia malmeana against Anticarsia gemmatalis.[Pubmed:18380460]
J Agric Food Chem. 2008 Apr 23;56(8):2655-9.
The insecticidal activity of hexane extracts from the roots and leaves of Aristolochia malmeana was evaluated against Anticarsia gemmatalis larvae by topical application. Extract from the roots was the most active and caused 50% mortality in larvae at 308.4 microg/microL. From this extract, a clerodane diterpene, (-)-Kolavenic acid, and three lignans, (-)-kusunokinin, (-)-hinokinin, and (8 S,8' R,9 S)-cubebin, were isolated by chromatography and partition procedures and then evaluated for their insecticidal activities either individually or in pairs. (-)-Kusunokinin showed higher activity against A. gemmatalis (LD10=9.3, LD50=230.1 microg/microL) than the crude extract, and its activity was dose-dependent, whereas the other constituents did not exhibit any significant activity. Together with (-)-kusunokinin and (-)-hinokinin, (-)-copalic acid, (-)-2-oxoKolavenic acid, (-)- ent-6-beta-hydroxy-copalic acid, (8 R,8' R,9 R)- and (8 R,8' R,9 S)-cubebins, (-)-fargesin, and (-)-phillygenin were isolated from the hexane extract of the leaves. The compounds were identified on the basis of spectroscopic analysis.
(-)-kolavenic Acid.[Pubmed:21202625]
Acta Crystallogr Sect E Struct Rep Online. 2008 May 17;64(Pt 6):o1114.
In the two, almost identical, mol-ecules in the asymmetric unit of the title compound [systematic name: (E)-3-methyl-5-(1,2,4a,5-tetra-methyl-1,2,3,4,4a,7,8,8a-octa-hydro-naphthalen-1-yl)pent-2-enoic acid], C(20)H(32)O(2), the rings are trans fused. The cyclo-hexane ring has a chair conformation and the cyclo-hexene ring a distorted half-boat conformation. The two independent mol-ecules are connected into a dimer via O-Hcdots, three dots, centeredO hydrogen bonds. The dimers are associated into supra-molecular chains along c via C-Hcdots, three dots, centeredO contacts.


