PratorimineCAS# 88660-12-2 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 88660-12-2 | SDF | Download SDF |
| PubChem ID | 181937 | Appearance | Powder |
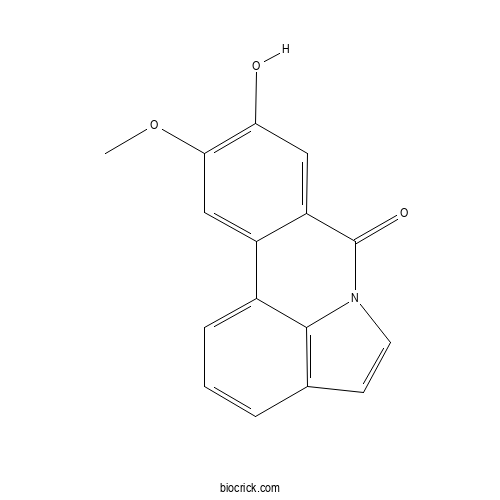
| Formula | C16H11NO3 | M.Wt | 265.26 |
| Type of Compound | Alkaloids | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | 5-hydroxy-4-methoxy-9-azatetracyclo[7.6.1.02,7.012,16]hexadeca-1(15),2,4,6,10,12(16),13-heptaen-8-one | ||
| SMILES | COC1=C(C=C2C(=C1)C3=CC=CC4=C3N(C2=O)C=C4)O | ||
| Standard InChIKey | LAKWSSVAGFQTAY-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C16H11NO3/c1-20-14-8-11-10-4-2-3-9-5-6-17(15(9)10)16(19)12(11)7-13(14)18/h2-8,18H,1H3 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Pratorimine Dilution Calculator

Pratorimine Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.7699 mL | 18.8494 mL | 37.6989 mL | 75.3977 mL | 94.2472 mL |
| 5 mM | 0.754 mL | 3.7699 mL | 7.5398 mL | 15.0795 mL | 18.8494 mL |
| 10 mM | 0.377 mL | 1.8849 mL | 3.7699 mL | 7.5398 mL | 9.4247 mL |
| 50 mM | 0.0754 mL | 0.377 mL | 0.754 mL | 1.508 mL | 1.8849 mL |
| 100 mM | 0.0377 mL | 0.1885 mL | 0.377 mL | 0.754 mL | 0.9425 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Fibraurin
Catalog No.:BCX0210
CAS No.:25254-84-6
- 1-Epideacetylbowdensine
Catalog No.:BCX0209
CAS No.:101219-55-0
- Copalic acid
Catalog No.:BCX0208
CAS No.:24470-48-2
- Diplacone
Catalog No.:BCX0207
CAS No.:73676-38-7
- 14,15-Dinorcleroda-3,11E-dien-13-one
Catalog No.:BCX0206
CAS No.:1186523-96-5
- Clauszoline B
Catalog No.:BCX0205
CAS No.:185508-03-6
- Kolavenic acid
Catalog No.:BCX0204
CAS No.:25436-90-2
- Crinine
Catalog No.:BCX0203
CAS No.:510-67-8
- 10-Hydroxyoleuropein
Catalog No.:BCX0202
CAS No.:84638-44-8
- Denticulatain E
Catalog No.:BCX0201
CAS No.:1919050-76-2
- Gnetuhainin N
Catalog No.:BCX0200
CAS No.:337464-99-0
- 4α-Hydroxybisabola-2,10-diene-1,9-dione
Catalog No.:BCX0199
CAS No.:1627567-79-6
- Gnetuhainin O
Catalog No.:BCX0212
CAS No.:337464-95-6
- (2R,3S)-Pterosin C
Catalog No.:BCX0213
CAS No.:68399-17-7
- Atalafoline
Catalog No.:BCX0214
CAS No.:59-49-4
- 3'-Hydroxyliquiritin
Catalog No.:BCX0215
CAS No.:1442113-42-9
- 25,26,27-Trinor-3α-hydroxycycloartan-24-oic acid
Catalog No.:BCX0216
CAS No.:1300747-31-2
- Cyclo(L-Leu-L-Pro)
Catalog No.:BCX0217
CAS No.:2873-36-1
- Demethylmangostanin
Catalog No.:BCX0218
CAS No.:2289591-37-1
- Cyclo(L-Pro-L-Val)
Catalog No.:BCX0219
CAS No.:2854-40-2
- Cyclo(L-Pro-L-Ile)
Catalog No.:BCX0220
CAS No.:57089-60-8
- Fornicin A
Catalog No.:BCX0221
CAS No.:908588-41-0
- 3β,5α-Dihydroxystigmastan-6-one
Catalog No.:BCX0222
CAS No.:55051-78-0
- 4-(1-Ethoxy-2-hydroxyethyl)benzene-1,2-diol
Catalog No.:BCX0223
CAS No.:1190632-33-7
Antibacterial agents from the leaves of Crinum purpurascens herb (Amaryllidaceae).[Pubmed:21503179]
Afr Health Sci. 2009 Dec;9(4):264-9.
BACKGROUND: Typhoid fevers and urogenital infections continue to be serious health problems in developing countries. In our search for therapeutic agents from natural sources with potential for the treatment of typhoid fevers and urogenital infections, extract and compounds were obtained from Crinum purpurascens and tested. METHODS: Two alkaloids (4,5-ethano-9,10-methylenedioxy-7-phenanthridone or hippadine (1) and 4,5-ethano-9-hydroxy-10-methoxy-7-phenanthridone or Pratorimine (2)) and one steroid (a-D-glucopyranoside of sitosterol (3)) were isolated from the CH(2)Cl(2)/MeOH (1:1) leaf extract of Crinum purpurascens and screened for antibacterial activity using both agar diffusion and broth dilution techniques. RESULTS: For the CH(2)Cl(2)/MeOH extract, the MIC values obtained were 3 mg/ml (against P. aeruginosa), 4 mg/ml (against E. coli, K. pneumoniae and S. aureus) and 6 mg/ml (against S. typhi and S. paratyphi B), whereas the MBC values varied between 7 and 12 mg/ml. For compound 1, the MIC values varied between 200 and 250 microg/ml, whereas the MBC value was 300 microg/ml against all the bacteria strains used. Compound 2 did not show any antimicrobial activity against these bacteria strains. For compound 3, the MIC values varied between 250 and 300 microg/ml, whereas the MBC values were 300 microg/ml (against S. typhi and S. paratyphi B) and > 300 microg/ml (against the other bacteria strains). CONCLUSION: These data suggest that C. purpurascens leaf extract contains antibacterial agents which could be used in the treatment of typhoid fevers and urogenital infections.
Cytotoxic alkaloids and a flavan from the bulbs of Crinum asiaticum var. japonicum.[Pubmed:11558618]
Chem Pharm Bull (Tokyo). 2001 Sep;49(9):1217-9.
A new pyrrolophenanthridone alkaloid, criasiaticidine A (1), was isolated from the bulbs of Crinum asiaticum var. japonicum, together with Pratorimine (2), lycorine (3) and 4'-hydroxy-7-methoxyflavan (4). The structure of the new alkaloid was determined to be 4,5-etheno-9,10-dihydroxy-6-phenanthridone by spectroscopic means. The cytotoxicity of the isolated compounds 1-4 was evaluated in vitro against Meth-A (mouse sarcoma) and Lewis lung carcinoma (mouse lung carcinoma) tumor cell lines. Furthermore, 3 was examined for in vivo antitumor activity with LLC tumor cells.


