CrocetindialCAS# 502-70-5 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 502-70-5 | SDF | Download SDF |
| PubChem ID | 25201897.0 | Appearance | Powder |
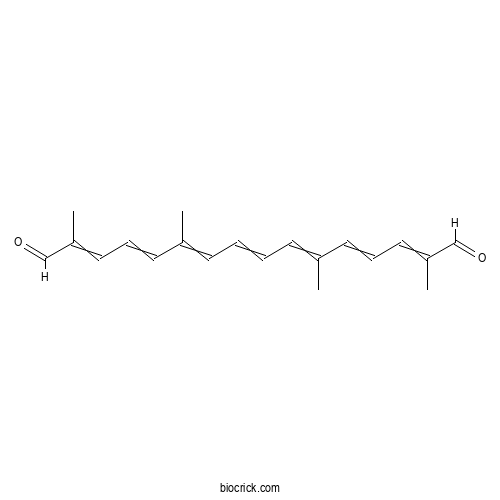
| Formula | C20H24O2 | M.Wt | 296.41 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | 2,6,11,15-tetramethylhexadeca-2,4,6,8,10,12,14-heptaenedial | ||
| SMILES | CC(=CC=CC=C(C)C=CC=C(C)C=O)C=CC=C(C)C=O | ||
| Standard InChIKey | YHCIKUXPWFLCFN-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C20H24O2/c1-17(11-7-13-19(3)15-21)9-5-6-10-18(2)12-8-14-20(4)16-22/h5-16H,1-4H3 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Crocetindial Dilution Calculator

Crocetindial Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.3737 mL | 16.8685 mL | 33.7371 mL | 67.4741 mL | 84.3426 mL |
| 5 mM | 0.6747 mL | 3.3737 mL | 6.7474 mL | 13.4948 mL | 16.8685 mL |
| 10 mM | 0.3374 mL | 1.6869 mL | 3.3737 mL | 6.7474 mL | 8.4343 mL |
| 50 mM | 0.0675 mL | 0.3374 mL | 0.6747 mL | 1.3495 mL | 1.6869 mL |
| 100 mM | 0.0337 mL | 0.1687 mL | 0.3374 mL | 0.6747 mL | 0.8434 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Gymnoside VII
Catalog No.:BCX1363
CAS No.:899430-07-0
- Blestriarene B
Catalog No.:BCX1362
CAS No.:127211-03-4
- 6-Benzoylheteratisine
Catalog No.:BCX1361
CAS No.:99759-48-5
- Ilexoside O
Catalog No.:BCX1360
CAS No.:136552-23-3
- Neokurarinol
Catalog No.:BCX1359
CAS No.:52483-00-8
- Cantleyoside
Catalog No.:BCX1358
CAS No.:32455-46-2
- 6""-apiosyl sec-O-glucosylhamaudol
Catalog No.:BCX1357
CAS No.:2254096-95-0
- (3R)-dihydroarteannuin B
Catalog No.:BCX1356
CAS No.:87206-33-5
- Eugenitin
Catalog No.:BCX1355
CAS No.:480-12-6
- Ajugamacrin B
Catalog No.:BCX1354
CAS No.:123313-59-7
- Kaempferol 3-O-β-D-glucopyranosyl-(1-2)-α-L-rhamnopyranoside
Catalog No.:BCX1353
CAS No.:142451-65-8
- Umbelliprenine
Catalog No.:BCX1352
CAS No.:23838-17-7
- Morroniaglycone
Catalog No.:BCX1365
CAS No.:1644061-02-8
- Ethyl (2E,4Z)-deca-2,4-dienoate
Catalog No.:BCX1366
CAS No.:3025-30-7
- Myristoleic acid
Catalog No.:BCX1367
CAS No.:544-64-9
- Elaidic acid methyl ester
Catalog No.:BCX1368
CAS No.:1937-62-8
- Cis-11-Eicosenoic acid
Catalog No.:BCX1369
CAS No.:5561-99-9
- Octadec-11-enoic acid
Catalog No.:BCX1370
CAS No.:693-72-1
- Tridecanoic acid
Catalog No.:BCX1371
CAS No.:638-53-9
- Methyl tridecanoate
Catalog No.:BCX1372
CAS No.:1731-88-0
- Pentadecanoic acid
Catalog No.:BCX1373
CAS No.:1002-84-2
- Heptadecanoic acid
Catalog No.:BCX1374
CAS No.:506-12-7
- Methyl heptadecanoate
Catalog No.:BCX1375
CAS No.:1731-92-6
- Nonadecanoic acid
Catalog No.:BCX1376
CAS No.:646-30-0
Carotenoid radical ions: A laser flash photolysis study.[Pubmed:32980657]
J Photochem Photobiol B. 2020 Nov;212:112023.
Laser excitation of a single precursor, namely 2-hydroxy-4'-(2-hydroxyethoxy)-2-methylpropiophenone (HHEMP), has been used for generating the radical cations and radical anions of various carotenoids in methanol. In the presence of oxygen, laser excitation of HHEMP undergoes an efficient alpha-cleavage reaction (Norrish type I) to form acyl radicals, which react with O(2), in a nearly diffusion-controlled reaction, to form their corresponding strong oxidizing acylperoxyl radicals (RO(2)(*)) (E = ~1.1 V (v SHE)), which are capable of oxidizing almost all carotenoids. Under argon-saturated conditions and in the presence of strong base (0.01 M NaOH or tetrabutylammonium hydroxide (TBAOH)), the initially formed 2-hydroxy-2-propyl radical (ACH(*)), generated after LFP of HHEMP, is deprotonated to form the strong reducing acetone ketyl radical (AC(*-)) (E acetone/ AC(*-) = -2.1 V (v SHE)), which is capable of reducing all carbonyl-containing carotenoids. To validate this new proposed approach, retinal and beta-apo-8'-carotenal (APO), with known spectroscopic data, were investigated in methanol, acetonitrile and tetrahydrofuran (THF). In addition, the radical ions of newly investigated carotenoids, namely 4-oxo-beta-apo-15'-carotenoic acid (4-oxo-15'), Crocetindial, 4-oxo-beta-apo-10'-carotenoic acid ethyl ester (4-oxo-10') and 4-oxo-beta-apo-8'-carotenoic acid ethyl ester (4-oxo-8') have been reported. Moreover, the scope of this approach has been extended to investigate the radical ions of chlorophyll b.
The intramolecular charge transfer state in carbonyl-containing polyenes and carotenoids.[Pubmed:20825184]
J Phys Chem B. 2010 Sep 30;114(38):12416-26.
Numerous femtosecond time-resolved optical spectroscopic experiments have reported that the lifetime of the low-lying S(1) state of carbonyl-containing polyenes and carotenoids decreases with increasing solvent polarity. The effect becomes even more pronounced as the number of double bonds in the conjugated pi-electron system decreases. The effect has been attributed to an intramolecular charge transfer (ICT) state coupled to S(1), but it is still not clear what the precise molecular nature of this state is, and how it is able to modulate the spectral and dynamic properties of polyenes and carotenoids. In this work, we examine the nature of the ICT state in three substituted polyenes: Crocetindial, which contains two terminal, symmetrically substituted carbonyl groups in conjugation with the pi-electron system, 8,8'-diapocarotene-8'-ol-8-al, which has one terminal conjugated carbonyl group and one hydroxyl group, and 8,8'-diapocarotene-8,8'-diol, which has two terminal, symmetrically positioned, hydroxyl groups but no carbonyls. Femtosecond time-resolved optical spectroscopic experiments on these molecules reveal that only the asymmetrically substituted 8,8'-diapocarotene-8'-ol-8-al exhibits any substantial effect of solvent on the excited state spectra and dynamics. The data are interpreted using molecular orbital theory which shows that the ICT state develops via mixing of the low-lying S(1) (2(1)A(g)-like) and S(2) (1(1)B(u)-like) excited singlet states to form a resultant state that preferentially evolves in polar solvent and exhibits a very large ( approximately 25 D) dipole moment. Molecular dynamics calculations demonstrate that the features of the ICT state are present in approximately 20 fs.
A short and efficient synthesis of crocetin-dimethylester and crocetindial.[Pubmed:14604394]
J Org Chem. 2003 Nov 14;68(23):9126-8.
In this paper we describe an efficient six-step synthesis of crocetin-dimethylester that could be further reduced to a "four-step" synthesis through the use of in situ procedures. The simplicity of the whole process, the ready availability of starting materials, and the high overall yield render this strategy a very attractive synthesis of this very important compound, which is the key intermediate for the synthesis of several carotenoids and other polyene natural products.


