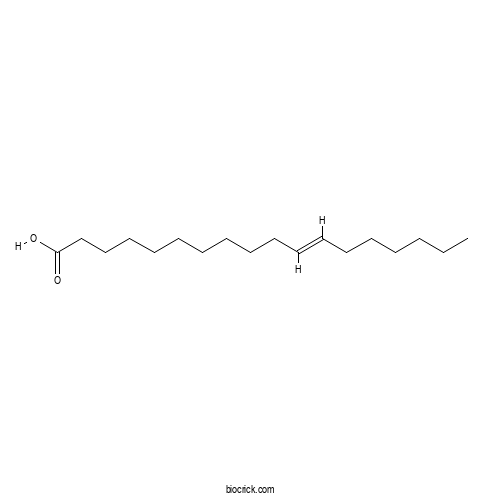
Octadec-11-enoic acidCAS# 693-72-1 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 693-72-1 | SDF | Download SDF |
| PubChem ID | 5281127.0 | Appearance | Powder |
| Formula | C18H34O2 | M.Wt | 282.47 |
| Type of Compound | Aliphatics | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | (E)-octadec-11-enoic acid | ||
| SMILES | CCCCCCC=CCCCCCCCCCC(=O)O | ||
| Standard InChIKey | UWHZIFQPPBDJPM-BQYQJAHWSA-N | ||
| Standard InChI | InChI=1S/C18H34O2/c1-2-3-4-5-6-7-8-9-10-11-12-13-14-15-16-17-18(19)20/h7-8H,2-6,9-17H2,1H3,(H,19,20)/b8-7+ | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Octadec-11-enoic acid Dilution Calculator

Octadec-11-enoic acid Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.5402 mL | 17.701 mL | 35.402 mL | 70.804 mL | 88.505 mL |
| 5 mM | 0.708 mL | 3.5402 mL | 7.0804 mL | 14.1608 mL | 17.701 mL |
| 10 mM | 0.354 mL | 1.7701 mL | 3.5402 mL | 7.0804 mL | 8.8505 mL |
| 50 mM | 0.0708 mL | 0.354 mL | 0.708 mL | 1.4161 mL | 1.7701 mL |
| 100 mM | 0.0354 mL | 0.177 mL | 0.354 mL | 0.708 mL | 0.885 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Cis-11-Eicosenoic acid
Catalog No.:BCX1369
CAS No.:5561-99-9
- Elaidic acid methyl ester
Catalog No.:BCX1368
CAS No.:1937-62-8
- Myristoleic acid
Catalog No.:BCX1367
CAS No.:544-64-9
- Ethyl (2E,4Z)-deca-2,4-dienoate
Catalog No.:BCX1366
CAS No.:3025-30-7
- Morroniaglycone
Catalog No.:BCX1365
CAS No.:1644061-02-8
- Crocetindial
Catalog No.:BCX1364
CAS No.:502-70-5
- Gymnoside VII
Catalog No.:BCX1363
CAS No.:899430-07-0
- Blestriarene B
Catalog No.:BCX1362
CAS No.:127211-03-4
- 6-Benzoylheteratisine
Catalog No.:BCX1361
CAS No.:99759-48-5
- Ilexoside O
Catalog No.:BCX1360
CAS No.:136552-23-3
- Neokurarinol
Catalog No.:BCX1359
CAS No.:52483-00-8
- Cantleyoside
Catalog No.:BCX1358
CAS No.:32455-46-2
- Tridecanoic acid
Catalog No.:BCX1371
CAS No.:638-53-9
- Methyl tridecanoate
Catalog No.:BCX1372
CAS No.:1731-88-0
- Pentadecanoic acid
Catalog No.:BCX1373
CAS No.:1002-84-2
- Heptadecanoic acid
Catalog No.:BCX1374
CAS No.:506-12-7
- Methyl heptadecanoate
Catalog No.:BCX1375
CAS No.:1731-92-6
- Nonadecanoic acid
Catalog No.:BCX1376
CAS No.:646-30-0
- Ethyl palmitoleate
Catalog No.:BCX1377
CAS No.:56219-10-4
- Palmitoleic acid
Catalog No.:BCX1378
CAS No.:373-49-9
- Arachidic acid
Catalog No.:BCX1379
CAS No.:506-30-9
- Ethyl Arachidonate
Catalog No.:BCX1380
CAS No.:1808-26-0
- Methyl arachidonate
Catalog No.:BCX1381
CAS No.:2566-89-4
- Ethyl oleate
Catalog No.:BCX1382
CAS No.:111-62-6
Amelioration of obesity induction by a high-fat diet and related inflammation by Phasa fish (Setipinna phasa) oil in BALB/c mice.[Pubmed:38505970]
J Appl Biomed. 2024 Mar;22(1):49-58.
We have extracted and characterized Phasa fish (Setipinna phasa) oil for the first time to evaluate the anti-obesity and related anti-inflammatory effects on obese mice. Inbred male albino BALB/c mice were segregated into three categories: control (C), Obese control group (OC), and Phasa fish oil treated group (TX). To establish the potentiality of Setipinna phasa oil for its anti-obesity and anti-inflammatory properties, it was extracted and characterized using GC-MS method. To evaluate the anti-obesity effect, different parameters were considered, such as body weight, lipid composition, obesity, and obesity associated inflammation. The physicochemical characteristics of Phasa fish oil revealed that the oil quality was good because acid value, peroxide value, p-anisidine value, Totox value, refractive index, and saponification value were within the standard value range. The GC-MS study explored the presence of fatty acids beneficial to health such as Hexadec-9-enoic acid; Octadec-11-enoic acid; EPA, DHA, Methyl Linolenate, etc. The application of Setipinna phasa oil on the treated mice group acutely lowered body weight and serum lipid profile compared to the obese group. In connection with this, leptin, FAS, and pro-inflammatory cytokines TNF-alpha genes expression were downregulated in the treated group compared to the obese group. The Phasa oil treated group had an elevated expression of PPAR-alpha, adiponectin, LPL gene, and anti-inflammatory markers IL-10 and IL-1Ra compared to the obese group. This study suggests that Phasa fish oil, enriched with essential fatty acid, might be used as an anti-obesity and anti-inflammatory supplement.
Network pharmacology‒based analysis of marine cyanobacteria derived bioactive compounds for application to Alzheimer's disease.[Pubmed:37927608]
Front Pharmacol. 2023 Oct 19;14:1249632.
In recent years, the Alzheimer's disease (AD) epidemic has become one of the largest global healthcare crises. Besides, the available systemic therapies for AD are still inadequate. Due to the insufficient therapeutic options, new treatment strategies are urgently needed to achieve a satisfactory therapeutic effect. Marine bio-resources have been accepted as one of the most economically viable and sustainable sources with potential applications for drug discovery and development. In this study, a marine cyanobacteria-Synechococcus sp. XM-24 was selected as the object of research, to systematically investigate its therapeutic potential mechanisms for AD. The major active compounds derived from the Synechococcus sp. biomass were identified via pyrolysis-gas chromatography-mass spectrometry (GC-MS), and 22 compounds were identified in this strain. The most abundant chemical compounds was (E)-Octadec-11-enoic acid, with the peak area of 30.6%. Follow by tridecanoic acid, 12-methyl- and hexadecanoic acid, with a peak area of 23.26% and 18.23%, respectively. GC-MS analysis also identified indolizine, isoquinoline, 3,4-dihydro- and Phthalazine, 1-methyl-, as well as alkene and alkane from the strain. After the chemical toxicity test, 10 compounds were finally collected to do the further analysis. Then, network pharmacology and molecular docking were adopted to systematically study the potential anti-AD mechanism of these compounds. Based on the analysis, the 10 Synechococcus-derived active compounds could interact with 128 related anti-AD targets. Among them, epidermal growth factor receptor (EGFR), vascular endothelial growth factor A (VEGFA) and mitogen-activated protein kinase 3 (MAPK3) were the major targets. Furthermore, the compounds N-capric acid isopropyl ester, (E)-Octadec-11-enoic acid, and 2H-Pyran-2,4(3H)-dione, dihydro-6-methyl- obtained higher degrees in the compounds-intersection targets network analysis, indicating these compounds may play more important role in the process of anti-AD. In addition, Kyoto Encyclopedia of Genes and Genomes (KEGG) enrichment analysis showed that these active compounds exert the anti-AD effects mainly through PI3K-Akt signaling pathway, neuroactive ligand-receptor interaction and ras signaling pathway. Our study identified Synechococcus-derived bioactive compounds have the potential for application to AD by targeting multiple targets and related pathways, which will provide a foundation for future research on applications of marine cyanobacteria in the functional drug industry.
Molecular effects of the consumption of margarine and butter varying in trans fat composition: a parallel human intervention study.[Pubmed:35982449]
Lipids Health Dis. 2022 Aug 18;21(1):74.
BACKGROUND: Whereas the dietary intake of industrial trans fatty acids (iTFA) has been specifically associated with inflammation, cardiovascular disease, and type 2 diabetes, understanding the impact of dietary fats on human health remains challenging owing to their complex composition and individual effects of their lipid components on metabolism. The aim of this study is to profile the composition of blood, measured by the fatty acid (FAs) profile and untargeted metabolome of serum and the transcriptome of blood cells, in order to identify molecular signatures that discriminate dietary fat intakes. METHODS: In a parallel study, the molecular effects of consuming dairy fat containing ruminant TFA (rTFA) or margarine containing iTFA were investigated. Healthy volunteers (n = 42; 45-69 y) were randomly assigned to diets containing margarine without TFA as major source of fat (wTFA control group with 0.4 g TFA per 100 g margarine), margarine with iTFA (iTFA group with 4.1 g TFA per 100 g margarine), or butter with rTFA (rTFA group with 6.3 g TFA per 100 g butter) for 4 weeks. The amounts of test products were individually selected so that fat intake contributed to 30-33% of energy requirements and TFA in the rTFA and iTFA groups contributed to up to 2% of energy intake. Changes in fasting blood values of lipid profiles (GC with flame-ionization detection), metabolome profiles (LC-MS, GC-MS), and gene expression (microarray) were measured. RESULTS: Eighteen FAs, as well as 242 additional features measured by LC-MS (185) and GC-MS (54) showed significantly different responses to the diets (P(FDR-adjusted) < 0.05), mainly distinguishing butter from the margarine diets while gene expression was not differentially affected. The most abundant TFA in the butter, i.e. TFA containing (E)-Octadec-11-enoic acid (C18:1 t11; trans vaccenic acid), and margarines, i.e. TFA containing (E)-octadec-9-enoic acid (C18:1 t9; elaidic acid) were reflected in the significantly different serum levels of TFAs measured after the dietary interventions. CONCLUSIONS: The untargeted serum metabolome differentiates margarine from butter intake although the identification of the discriminating features remains a bottleneck. The targeted serum FA profile provides detailed information on specific molecules differentiating not only butter from margarine intake but also diets with different content of iTFAs in margarine. TRIAL REGISTRATION: ClinicalTrials.gov NCT00933322.
Characterization of Bitter and Astringent Off-Taste Compounds in Potato Fibers.[Pubmed:32930579]
J Agric Food Chem. 2020 Oct 14;68(41):11524-11534.
Applying the sensomics approach, a combination of activity-guided fractionation and taste dilution analysis (TDA) followed by high-performance liquid chromatography-tandem mass spectrometry (HPLC-MS/MS), ultrahigh-performance liquid chromatography time-of-flight mass spectrometry (UHPLC-TOF-MS), and one-dimensional and two-dimensional nuclear magnetic resonance spectroscopy (1D/2D NMR) allowed the elucidation of key off-taste compounds in potato dietary fiber isolates. Previously already having been described as off-taste compounds in potato tubers, saponins alpha-chaconine and alpha-solanine were shown to be also major contributors to overall off-taste in potato fiber isolates. Moreover, fatty acids as well as fatty acid oxidation products, namely, E-9,10,13-trihydroxy-Octadec-11-enoic acid as well as newly identified compounds hexadecyl(E/Z)-ferulate and octadecyl(E/Z)-ferulate, were shown to be key inducers to off-taste in the isolates, exhibiting taste recognition thresholds between 18 and 981 mumol/L. This paper demonstrates the isolation, structure determination, quantitation as well as sensory attributes of these key off-taste compounds.
GC-MS structural characterization of fatty acids from marine aerobic anoxygenic phototrophic bacteria.[Pubmed:15825835]
Lipids. 2005 Jan;40(1):97-108.
The FA composition of 12 strains of marine aerobic anoxygenic phototrophic bacteria belonging to the genera Erythrobacter, Roseobacter, and Citromicrobium was investigated. GC-MS analyses of different types of derivatives were performed to determine the structures of the main FA present in these organisms. All the analyzed strains contained the relatively rare 11-methyloctadec-12-enoic acid, and three contained 12-methyl-Octadec-11-enoic acid, which has apparently never been reported before. High amounts of the very unusual octadeca-5,11-dienoic acid were present in 9 of the 12 strains analyzed. A FA containing a furan ring was detected in three strains. Analytical data indicated that this FA was 10,13-epoxy-11-methyloctadeca-10,12-dienoic acid. A very interesting enzymatic peroxidation of the allylic carbon 10 of cis-vaccenic acid was observed in three strains. Deuterium labeling and GC-MS analyses enabled us to demonstrate that this enzymatic process involves the initial dioxygenase-mediated formation of 10-hydroperoxyoctadec-11(cis)-enoic acid, which is then isomerized to 10-hydroperoxyoctadec-11(trans)-enoic acid and converted to the corresponding hydroxyacids and oxoacids. Different biosynthetic pathways were proposed for these different compounds.
Polar lipids and fatty acids of Pseudomonas cepacia.[Pubmed:2912494]
Biochim Biophys Acta. 1989 Jan 23;1001(1):60-7.
The polar lipids and fatty acids produced by the reference strains for seven different O serogroups of Pseudomonas cepacia have been identified. Similar results were obtained for all strains. Contrary to a previous report, the only significant phospholipids in this species are phosphatidylethanolamine and bis(phosphatidyl)glycerol, which contributed 57-83% and 17-43%, respectively, of the total lipid phosphorus. The former lipid was found as two chromatographically distinct fractions. In the less polar fraction and in bis(phosphatidyl)glycerol, the major fatty acids were hexadecanoic acid, cis-9,10-methylenehexadecanoic acid, cis-Octadec-11-enoic acid, and cis-11,12-methyleneoctadecanoic acid. In the more polar fraction of phosphatidylethanolamine, the fatty acid in one position is a 2-hydroxy acid, mainly 2-hydroxyhexadecenoic acid, 2-hydroxyhexadecanoic acid, 2-hydroxyoctadecenoic acid, or 2-hydroxymethyleneoctadecanoic acid. Compared with other phospholipids, this fraction of phosphatidylethanolamine was depleted in cis-9,10-methylenehexadecanoic acid. Each strain also produced two ornithine amide lipids. In the major lipid, 3-hydroxyhexadecanoic acid was amide-bound to the alpha-amino group and was itself probably esterified by a 2-hydroxy acid, mainly 2-hydroxyoctadecenoic acid or the derived cyclopropane acid. In the minor ornithine amide lipid, the ester-bound acids were mainly methyleneoctadecanoic acid and hexadecanoic acid. The unusual lipid profiles of P. cepacia are of chemotaxonomic interest.
Isolation and characterization of an ornithine-containing lipid from Paracoccus denitrificans.[Pubmed:7379785]
Eur J Biochem. 1980 Apr;105(2):267-74.
The isolation and characterization of an ornithine-containing lipid from various strains of Paracoccus denitrificans (grown heteretrophically and autotrophically) is reported. The structure of this aminolipid was found to be H2N--CH2--(CH2)2--CH[NH--CO--CH2--CH(O--CO--R1)--R2]--CO2H, where R1 predominantly represents the residue of Octadec-11-enoic acid, R2 the residue of a 3-hydroxyeicos-13-enoic acid. In addition, the major phospholipids (phosphatidylcholine, phosphatidylethanolamine, phosphatidylglycerol) were isolated and characterized.
Enzymic reactions of fatty acid hydroperoxides in extracts of potato tuber. II. Conversion of 9- and 13-hydroperoxy-octadecadienoic acids to monohydroxydienoic acid, epoxyhydroxy- and trihydroxymonoenoic acid derivatives.[Pubmed:63]
Biochim Biophys Acta. 1975 Nov 21;409(2):157-71.
1. Crude extracts and partially purified enzyme preparations from potato tubers catalyse, at pH 5-7, the conversion of linoleic acid hydroperoxides to a range of oxygenated fatty acid derivatives. 2. 9-D- and 13-L-hydroperoxide isomers are converted at similar rates to equivalent (isomeric) products. 3. The major products from the 13-hydroperoxide isomer were identified as the corresponding monohydroxydienoic acid derivative, threo-11-hydroxy-trans12,13-epoxy-octadec-cis9-enoic acid and 9,12,13-trihydroxy-octadec-trans10-enoic acid. The corresponding products from the 9-hydroperoxide were the monohydroxydienoic acid, 9,10-epoxy-11-hydroxy-octadec-12-enoic acid and 9,10,13-trihydroxy-Octadec-11-enoic acid. 4. No separation of activities forming the different products was achieved by partial purification of enzyme extracts. 5. Product formation was unaffected by EDTA, CN-, sulphydryl reagents or glutathione but was reduced by boiling the extracts. 6. This system is compared with the 9-hydroperoxide-specific enzymic formation of divinyl ether derivatives by potato extracts.


