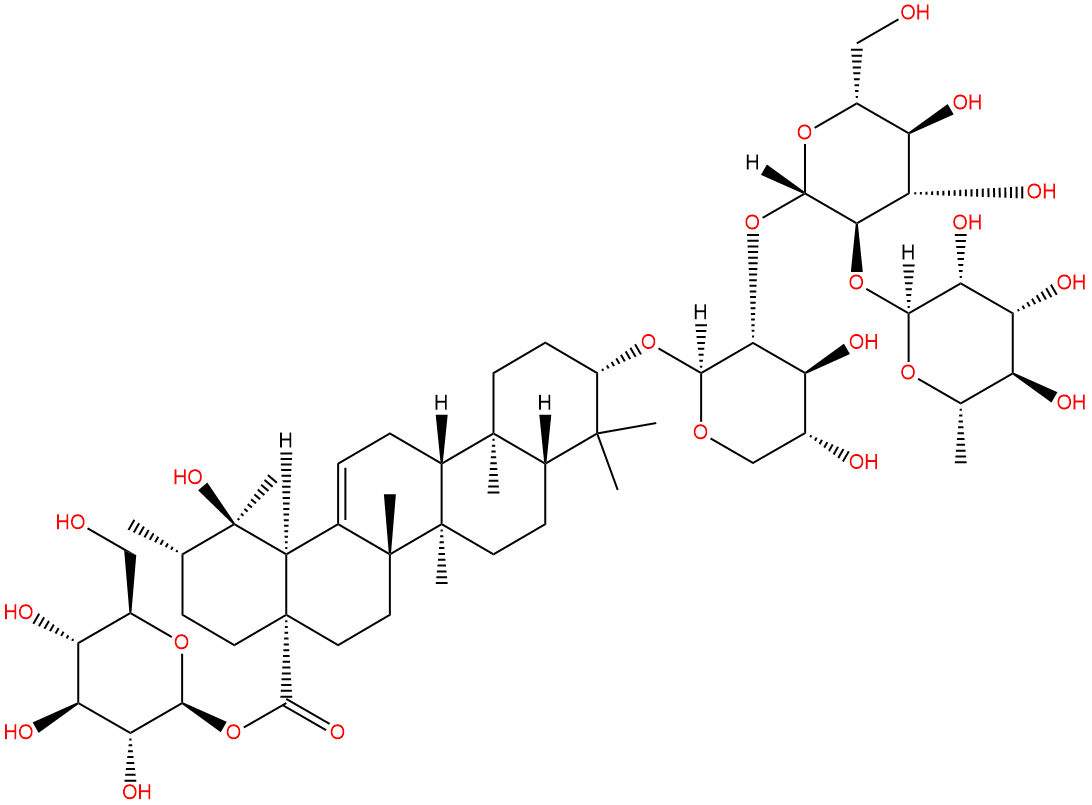
Ilexoside OCAS# 136552-23-3 |
Quality Control & MSDS
Package In Stock
Number of papers citing our products

| Cas No. | 136552-23-3 | SDF | File under preparation. |
| PubChem ID | N/A | Appearance | Powder |
| Formula | C53H86O22 | M.Wt | 1075.25 |
| Type of Compound | Steroids | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Ilexoside O Dilution Calculator

Ilexoside O Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 0.93 mL | 4.6501 mL | 9.3002 mL | 18.6003 mL | 23.2504 mL |
| 5 mM | 0.186 mL | 0.93 mL | 1.86 mL | 3.7201 mL | 4.6501 mL |
| 10 mM | 0.093 mL | 0.465 mL | 0.93 mL | 1.86 mL | 2.325 mL |
| 50 mM | 0.0186 mL | 0.093 mL | 0.186 mL | 0.372 mL | 0.465 mL |
| 100 mM | 0.0093 mL | 0.0465 mL | 0.093 mL | 0.186 mL | 0.2325 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Neokurarinol
Catalog No.:BCX1359
CAS No.:52483-00-8
- Cantleyoside
Catalog No.:BCX1358
CAS No.:32455-46-2
- 6""-apiosyl sec-O-glucosylhamaudol
Catalog No.:BCX1357
CAS No.:2254096-95-0
- (3R)-dihydroarteannuin B
Catalog No.:BCX1356
CAS No.:87206-33-5
- Eugenitin
Catalog No.:BCX1355
CAS No.:480-12-6
- Ajugamacrin B
Catalog No.:BCX1354
CAS No.:123313-59-7
- Kaempferol 3-O-β-D-glucopyranosyl-(1-2)-α-L-rhamnopyranoside
Catalog No.:BCX1353
CAS No.:142451-65-8
- Umbelliprenine
Catalog No.:BCX1352
CAS No.:23838-17-7
- Yuanamide
Catalog No.:BCX1351
CAS No.:102421-42-1
- O-Methylbulbocapnine
Catalog No.:BCX1350
CAS No.:2490-83-7
- Calycanthidine
Catalog No.:BCX1349
CAS No.:5516-85-8
- Volvalerenal E
Catalog No.:BCX1348
CAS No.:1247014-33-0
- 6-Benzoylheteratisine
Catalog No.:BCX1361
CAS No.:99759-48-5
- Blestriarene B
Catalog No.:BCX1362
CAS No.:127211-03-4
- Gymnoside VII
Catalog No.:BCX1363
CAS No.:899430-07-0
- Crocetindial
Catalog No.:BCX1364
CAS No.:502-70-5
- Morroniaglycone
Catalog No.:BCX1365
CAS No.:1644061-02-8
- Ethyl (2E,4Z)-deca-2,4-dienoate
Catalog No.:BCX1366
CAS No.:3025-30-7
- Myristoleic acid
Catalog No.:BCX1367
CAS No.:544-64-9
- Elaidic acid methyl ester
Catalog No.:BCX1368
CAS No.:1937-62-8
- Cis-11-Eicosenoic acid
Catalog No.:BCX1369
CAS No.:5561-99-9
- Octadec-11-enoic acid
Catalog No.:BCX1370
CAS No.:693-72-1
- Tridecanoic acid
Catalog No.:BCX1371
CAS No.:638-53-9
- Methyl tridecanoate
Catalog No.:BCX1372
CAS No.:1731-88-0
Pharmacokinetic and tissue distribution study of six saponins in the rat after oral administration of Ilex pubescens extract using a validated simultaneous UPLC-qTOF-MS/MS assay.[Pubmed:37148697]
J Pharm Biomed Anal. 2023 Sep 5;233:115431.
Ilex pubescens Hook. et Arn is a medicinal plant of the Ilex family that is mainly used for the treatment of cardiovascular diseases. Its main medicinal ingredients are total triterpenoid saponins (IPTS). However, the pharmacokinetics and tissue distribution of the main multi-triterpenoid saponins are lacking. This is the first report that demonstrates a sensitive ultra-performance liquid chromatography coupled with quadrupole time-of-flight tandem mass spectrometry (UPLC-qTOF-MS/MS) method for the quantification of ilexgenin A (C1), ilexsaponin A1 (C2), ilexsaponin B1 (C3), ilexsaponin B2 (C4), ilexsaponin B3 (DC1) and Ilexoside O (DC2) in rat plasma and various tissues of the heart, liver, spleen, lungs, kidney, brain, stomach, duodenum, jejunum, ileum, colon and thoracic aorta. The chromatographic separation was carried out on an Acquity HSS T3 UPLC column (2.1 x 100 mm, 1.8 microm, Waters, USA) with a mobile phase consisting of 0.1% (v/v) formic acid (A) and acetonitrile containing 0.1% (v/v) formic acid (B) at a flow rate of 0.25 mL/min. The MS/MS detection was performed by electrospray ionization (ESI) using selected ion monitoring (SIM) in negative scan mode. The developed quantification method showed good linearity over the concentration range of 10-2000 ng/mL for plasma and 25-5000 ng/mL for tissue homogenates with R(2) >/= 0.990. Lower limits of quantification (LLOQ) was 10 ng/mL in plasma and 25 ng/mL in tissue homogenates. The intra- and inter-day precision were less than 10.39%, and the accuracy was between - 1.03% and 9.13%. The extract recoveries, dilution integrity and matrix effect were well within satisfactory limits. Using the validated method, the pharmacokinetic parameters, including half-life, AUC, Cmax, CL, and MRT, of six triterpenoid saponins in rats after oral administration were provided by establishing their plasma concentration-time curves, while their absolute quantification in various tissues after oral administration was also determined at first, which provides a scientific basis for their clinical application.
Rapid profiling and pharmacokinetic studies of multiple potential bioactive triterpenoids in rat plasma using UPLC/Q-TOF-MS/MS after oral administration of Ilicis Rotundae Cortex extract.[Pubmed:29981874]
Fitoterapia. 2018 Sep;129:210-219.
Triterpenoids, the major bioactive ingredients of Ilicis Rotundae Cortex, contributes a significant cardiovascular protection activity. Although many studies about the total saponins have been reported, the absorption triterpenoids and pharmacokinetic behaviors were unclear. Thus, the present study aims to comprehensive elucidate the absorption triterpenoids and their pharmacokinetics in rats after oral administration the crude extract using UPLC/Q-TOF-MS/MS. A total of forty-two triterpenoids were successfully characterized from the rat plasma, and thirty-two of them were validated by the reference substances, while the others were tentatively identified based on the mass spectral fragmental patterns. Furthermore, the plasma concentrations of six absorption bioactive triterpenoids (rotundinoside C, Ilexoside O, pedunculoside, rotundic acid, rotundanonic acid and ilexgenin A) were simultaneously quantified by selected reaction monitoring in negative ionization mode. All analytes exhibited good linearity with correlation coefficients values greater than 0.99 and the LLOQ ranged from 1.2 to 3.2 ng/mL, and method validation for selectivity, precision, accuracy, recovery, matrix effect and stability were reckoned acceptable. The results were successfully applied for the multiple-component pharmacokinetic study of the six bioactive triterpenoids.
Study of Absorption Characteristics of the Total Saponins from Radix Ilicis Pubescentis in an In Situ Single-Pass Intestinal Perfusion (SPIP) Rat Model by Using Ultra Performance Liquid Chromatography (UPLC).[Pubmed:29104273]
Molecules. 2017 Nov 1;22(11):1867.
In contrast to the extensively reported therapeutic activities, far less attention has been paid to the intestinal absorption of the total saponins from Radix Ilicis Pubescentis (in Chinese Mao-Dong-Qing, MDQ). This study aimed to investigate the intestinal absorption characteristics of ilexgenin A (C1), ilexsaponin A1 (C2), ilexsaponin B1 (C3), ilexsaponin B2 (C4), ilexsaponin B3 (DC1), and Ilexoside O (DC2) when administrated with the total saponins from MDQ (MDQ-TS). An UPLC method for simultaneous determination of C1, C2, C3, C4, DC1, and DC2 in intestinal outflow perfusate was developed and validated. The absorption characteristics of MDQ-TS were investigated by evaluating the effects of intestinal segments, drug concentration, P-glycoprotein (P-gp) inhibitor (verapomil), endocytosis inhibitor (amantadine) and ethylene diamine tetraacetic acid (EDTA, tight junction modulator) on the intestinal transportation of MDQ-TS by using a single-pass intestinal perfusion (SPIP) rat model, and the influence of co-existing components on the intestinal transport of the six saponins was discussed. The results showed that effective apparent permeability (P(app)) of C1, C2, C3, C4, and DC2 administrated in MDQ-TS form had no segment-dependent changes at low and middle dosage levels. C1, C2, C3, D4, DC1, and DC2 administrated in MDQ-TS form all exhibited excellent transmembrane permeability with P(app) > 0.12 x 10(-2) cm.min(-1). Meanwhile, P(app) and effective absorption rate constant (K(a)) values for the most saponins showed concentration dependence and saturation characteristics. After combining with P-gp inhibitor of verapamil, P(app) of C2, C3, and DC1 in MDQ-TS group was significantly increased up to about 2.3-fold, 1.4-fold, and 3.4-fold, respectively in comparison to that of non-verapamil added group. Verapamil was found to improve the absorption of C2, C3, and DC1, indicating the involvement of an active transport mechanism in the absorption process. Compared with the non-amantadine added group, the absorption of C1, C2, C4, DC1, and DC2 were decreased by 40%, 71%, 31%, 53%, and 100%, respectively. P(app) for the six target compounds increased up to about 1.2-2.1-fold in comparison with the non-EDTA added, respectively. The gastrointestinal transport of MDQ-TS could be greatly promoted by EDTA, and inhibited by amantadine, implying that the intestinal absorption of MDQ-TS was by passive diffusion and endocytosis process. Compared with monomer administration group, the intestinal absorption of C3, C4, DC1, and DC2 was significantly improved by co-existing components in MDQ-TS, and the non-absorbable saponins of C4, DC1, and DC2 unexpectedly showed sufficient intestinal permeability with P(app) > 0.12 x 10(-2) cm.min(-1). This suggested that compounds orally administrated in TCM extract forms displayed unique intestinal absorption characteristics different from those of monomers, and the enhancing intestinal absorption of MDQ-TS reflected a holistic and specific view of traditional Chinese medicines (TCMs).
A new triterpene saponin from the roots of Ilex pubescens.[Pubmed:23092447]
Nat Prod Res. 2013 Aug;27(15):1343-7.
A new triterpene saponin, ilexoside P (1), along with three known triterpene saponins, Ilexoside O (2), ilexsaponin B3 (3) and ilexpublesnin A (4) was isolated from the roots of Ilex pubescens. Their chemical structures were elucidated on the basis of UV, IR, MS, and NMR spectroscopic analyses coupled with chemical degradation. In addition, the xanthine oxidase (XOD) inhibitory activity of the isolated saponins was reported. Compound 1 exhibited weak XOD inhibitory activity in the test.
Qualitative and quantitative analysis of the main constituents of Radix Ilicis Pubescentis by LC-coupled with DAD and ESI-MS detection.[Pubmed:20184013]
Nat Prod Commun. 2010 Jan;5(1):23-6.
A sensitive and selective high performance liquid chromatographic method coupled with DAD detection is presented for quality control of Radix Ilicis Pubescentis. By means of this analytical procedure the major individual constituents (Ilexoside O, ilexgenin A, ilexsaponin A1, ilexsaponin B1, liriodendin and acanthoside B) could be quantified simultaneously. LC-ESI-MS was applied for identification of the six compounds in the plant by comparing their m/z value and retention times with those of selected standards. For quantitative analysis, the extraction procedure and the extraction solvent were optimized in order to ensure the exhaustive extraction of the plant material. The HPLC conditions were evaluated and optimized for the exact quantification of all six individual compounds. Chromatographic separation was carried out on a C18 column using gradient elution with acetonitrile and 0.1% phosphoric acid as the mobile phase. Detection was carried out using a photodiode array detector. The calibration curves for determination of the six constituents showed good linearity over the investigated ranges (r2>0.999). Measurement of intra-day and inter-day variability (expressed as RSD value) was conducted to assess precisions of the method, and RSD (%) of intra- and inter-day variation were between 1.56-3.36% and 1.61-3.58%, respectively. The recoveries of the six compounds were between 96.4-102.2%, with RSD (%) values ranging from 1.7-3.8%. These validation results demonstrated the suitability of the method for the precise and accurate determination of the main constituents in Radix Ilicis Pubescentis. The method was successfully applied for quality evaluation of 12 batches of Radix Ilicis Pubescentis obtained from different regions of southern China. The contents of the six major constituents varied significantly due to their different origins, which can be used as an aid to assessing the quality of Radix Ilicis Pubescentis.


