CantleyosideCAS# 32455-46-2 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 32455-46-2 | SDF | Download SDF |
| PubChem ID | 12302405.0 | Appearance | Powder |
| Formula | C33H46O19 | M.Wt | 746.71 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
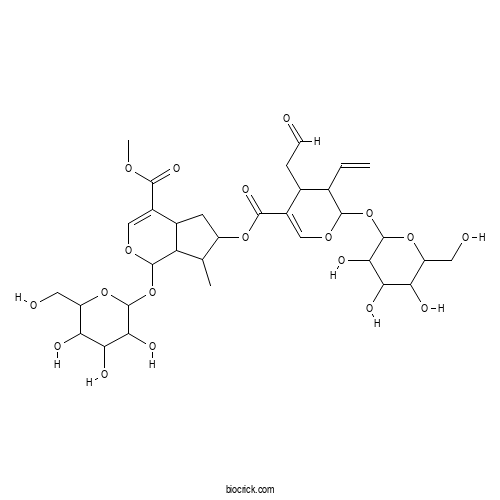
| Chemical Name | methyl 6-[3-ethenyl-4-(2-oxoethyl)-2-[3,4,5-trihydroxy-6-(hydroxymethyl)oxan-2-yl]oxy-3,4-dihydro-2H-pyran-5-carbonyl]oxy-7-methyl-1-[3,4,5-trihydroxy-6-(hydroxymethyl)oxan-2-yl]oxy-1,4a,5,6,7,7a-hexahydrocyclopenta[c]pyran-4-carboxylate | ||
| SMILES | CC1C(CC2C1C(OC=C2C(=O)OC)OC3C(C(C(C(O3)CO)O)O)O)OC(=O)C4=COC(C(C4CC=O)C=C)OC5C(C(C(C(O5)CO)O)O)O | ||
| Standard InChIKey | GXXXVFMBJGIYPK-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C33H46O19/c1-4-13-14(5-6-34)16(10-46-30(13)51-32-26(41)24(39)22(37)19(8-35)49-32)29(44)48-18-7-15-17(28(43)45-3)11-47-31(21(15)12(18)2)52-33-27(42)25(40)23(38)20(9-36)50-33/h4,6,10-15,18-27,30-33,35-42H,1,5,7-9H2,2-3H3 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Cantleyoside Dilution Calculator

Cantleyoside Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 1.3392 mL | 6.696 mL | 13.3921 mL | 26.7842 mL | 33.4802 mL |
| 5 mM | 0.2678 mL | 1.3392 mL | 2.6784 mL | 5.3568 mL | 6.696 mL |
| 10 mM | 0.1339 mL | 0.6696 mL | 1.3392 mL | 2.6784 mL | 3.348 mL |
| 50 mM | 0.0268 mL | 0.1339 mL | 0.2678 mL | 0.5357 mL | 0.6696 mL |
| 100 mM | 0.0134 mL | 0.067 mL | 0.1339 mL | 0.2678 mL | 0.3348 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- 6""-apiosyl sec-O-glucosylhamaudol
Catalog No.:BCX1357
CAS No.:2254096-95-0
- (3R)-dihydroarteannuin B
Catalog No.:BCX1356
CAS No.:87206-33-5
- Eugenitin
Catalog No.:BCX1355
CAS No.:480-12-6
- Ajugamacrin B
Catalog No.:BCX1354
CAS No.:123313-59-7
- Kaempferol 3-O-β-D-glucopyranosyl-(1-2)-α-L-rhamnopyranoside
Catalog No.:BCX1353
CAS No.:142451-65-8
- Umbelliprenine
Catalog No.:BCX1352
CAS No.:23838-17-7
- Yuanamide
Catalog No.:BCX1351
CAS No.:102421-42-1
- O-Methylbulbocapnine
Catalog No.:BCX1350
CAS No.:2490-83-7
- Calycanthidine
Catalog No.:BCX1349
CAS No.:5516-85-8
- Volvalerenal E
Catalog No.:BCX1348
CAS No.:1247014-33-0
- 8-Formylophiopogonone B
Catalog No.:BCX1347
CAS No.:1316224-74-4
- 5, 9-epi-Phlomiol
Catalog No.:BCX1346
CAS No.:1621908-70-0
- Neokurarinol
Catalog No.:BCX1359
CAS No.:52483-00-8
- Ilexoside O
Catalog No.:BCX1360
CAS No.:136552-23-3
- 6-Benzoylheteratisine
Catalog No.:BCX1361
CAS No.:99759-48-5
- Blestriarene B
Catalog No.:BCX1362
CAS No.:127211-03-4
- Gymnoside VII
Catalog No.:BCX1363
CAS No.:899430-07-0
- Crocetindial
Catalog No.:BCX1364
CAS No.:502-70-5
- Morroniaglycone
Catalog No.:BCX1365
CAS No.:1644061-02-8
- Ethyl (2E,4Z)-deca-2,4-dienoate
Catalog No.:BCX1366
CAS No.:3025-30-7
- Myristoleic acid
Catalog No.:BCX1367
CAS No.:544-64-9
- Elaidic acid methyl ester
Catalog No.:BCX1368
CAS No.:1937-62-8
- Cis-11-Eicosenoic acid
Catalog No.:BCX1369
CAS No.:5561-99-9
- Octadec-11-enoic acid
Catalog No.:BCX1370
CAS No.:693-72-1
Divergent Total Syntheses of Hetero-Oligomeric Iridoid Glycosides.[Pubmed:36607173]
Org Lett. 2023 Jan 20;25(2):347-352.
Divergent total syntheses of the hetero-oligomeric iridoid glycosides mainly found in Dipsacus asper were achieved. Thus, loganin (1), which is important as a monomer unit, was efficiently synthesized by stereoselective reductive cyclization using secologanin (2) as a substrate. Sequential condensation reactions of derivatives of 1 and 2 as monomer units led to the first enantioselective total syntheses of the heterooligomers Cantleyoside, (E)-aldosecologanin, dipsaperine, (3R, 5S)-5-carboxyvincosidic acid 22-loganin ester, and dipsanoside A.
The enhanced mitochondrial dysfunction by cantleyoside confines inflammatory response and promotes apoptosis of human HFLS-RA cell line via AMPK/Sirt 1/NF-kappaB pathway activation.[Pubmed:35364376]
Biomed Pharmacother. 2022 May;149:112847.
OBJECTIVE: Cantleyoside (CA) is a kind of iridoid glycosides in Pterocephalus hookeri (C. B. Clarke) Hoeck. The purpose of this study was to investigate the effects of CA on human rheumatoid arthritis fibroblast synovial cells (HFLS-RA). METHODS: Cell proliferation of HFLS-RA was assessed by CCK-8. ELISA was used to detect cytokines NO, TNF-alpha, IL-1beta/6, MCP-1, MMP-1/3/9 and metabolism-related ATPase activities and ATP levels. JC-1, DCFH-DA, Fluo-3 AM and Calcein AM probes were used to detect mitochondrial membrane potential (MMP), reactive oxygen species (ROS), Ca(2+) and mitochondrial permeability conversion pore (MPTP), respectively. Isolated mitochondria assay was used to detect mitochondrial swelling. Oxygen consumption rate (OCR), extracellular acidification rate (ECAR) and real-time ATP production were measured using a Seahorse analyzer. Apoptosis was detected by TUNEL and Hoechst staining. Western blot was used to detect the expressions of AMPK/p-AMPK, Sirt 1, IkappaBalpha, NF-kappaB p65/p-NF-kappaB p65, Bcl-2 and Bax. Cytoplasmic nuclear isolation was also performed to detect the translocation of NF-kappaB. RESULTS: CA significantly suppressed cell proliferation and the levels of NO, TNF-alpha, IL-1beta/6, MCP-1 and MMP-1/3/9 in HFLS-RA. In addition, CA promoted the apoptosis of HFLS-RA by increasing TUNEL and Hoechst positive cells and the ratio of Bax/Bcl-2. Inhibition of energy metabolism in HFLS-RA by CA reduced OCR, ECAR and real-time ATP generation rate. Importantly, CA promoted p-AMPK and Sirt 1 expression, inhibited IkappaBalpha degradation to reduce p-NF-kappaB and translocation. CONCLUSION: The results suggest that CA activates the AMPK/Sirt 1/NF-kappaB pathway by promoting mitochondrial dysfunction, thereby exerting anti-inflammatory and pro-apoptotic effects.
Roots and Leaf Extracts of Dipsacus fullonum L. and Their Biological Activities.[Pubmed:31936189]
Plants (Basel). 2020 Jan 8;9(1):78.
The aim of the study was to identify and evaluate the content of iridoids and phenolic compounds in the leaves and roots of Dipsacus fullonum L. They were identified and quantified by UPLC-PDA-MS/MS. Five iridoid compounds (loganic acid, loganin, sweroside, Cantleyoside, and sylvestroside III) were identified in Dipsacus fullonum L. leaves and roots. Seven phenolic acids and three flavones were identified in the leaves, and seven phenolic acids were detected in the roots. The leaves contained more iridoids and phenolic compounds than the roots. We also evaluated the antimicrobial (anti-bacterial and anti-yeast), antioxidant (ORAC methods), and antiacetylcholinesterase (AChE) activities of Dipsacus fullonum L. leaves and roots. Leaf extract demonstrated the strongest antioxidant activity, but roots showed stronger antiacetylcholinesterase activity than leaves. The study also confirmed antibacterial activity of root-derived compounds against Staphylococcus aureus DSM 799 and Escherichia coli ATCC 10536.
A new iridoid glycoside from the roots of Dipsacus asper.[Pubmed:22306831]
Molecules. 2012 Feb 3;17(2):1419-24.
A new iridoid glycoside, named loganic acid ethyl ester (1), together with five known compounds: chlorogenic acid (2), caffeic acid (3), loganin (4), Cantleyoside (5) and syringaresinol-4',4''-O-bis-beta-D-glucoside (6) were isolated from the roots of Dipsacus asper. The structure of compound 1 was elucidated on the basis of detailed spectroscopic analyses. Lignan is isolated from Dipsacaceae species for the first time. Compounds 1, 4 and 5 had moderate neuroprotective effects against the Abeta(2)(5)(-)(3)(5) induced cell death in PC12 cells.
Growth inhibiting activity of lipophilic extracts from Dipsacus sylvestris Huds. roots against Borrelia burgdorferi s. s. in vitro.[Pubmed:21901989]
Pharmazie. 2011 Aug;66(8):628-30.
Fresh first year roots from Dipsacus sylvestris HUDS. were extracted with 70% ethanol, ethyl acetate as well as dichloromethane. Extracts were solubilized in water (lipophilic extracts with addition of polysorbate 80) and tested for their activity against Borrelia burgdorferi sensu stricto in vitro during an eight-day period using amoxicillin as standard. The hydroethanolic extract showed no growth inhibition whereas significant growth inhibiting activity could be shown in the two less polar fractions for the first time. Strongest inhibition was found in the ethyl acetate extract. The effect of polysorbate 80 on bacterial growth was examined and found to be negligible. As the nature of bioactive constituents has not been clarified yet, a micellar electrokinetic capillary chromatography fingerprint analysis for a methanolic extract was applied including loganin, chlorogenic acid, Cantleyoside and caffeic acid as marker substances.
Monoterpenoid glucoindole alkaloids and iridoids from Pterocephalus pinardii.[Pubmed:20049748]
Magn Reson Chem. 2010 Mar;48(3):239-43.
A new secondary metabolite, pterocephaline, along with the known Cantleyoside, 7alpha-morroniside, 3beta,5alpha-tetrahydrodesoxycordifoline lactam, 5S-5-carboxyvincoside, sweroside, and loganin have been isolated from the aerial parts of P. pinardii (Dipsacaceae). Moreover, Cantleyoside-methyl-hemiacetal and Cantleyoside-dimethyl-acetal were obtained as seco-iridoid artifacts. The structures were elucidated by extensive spectroscopic methods including 1D-((1)H, (13)C and TOCSY) and 2D-NMR (DQF-COSY, HSQC and HMBC). Monoterpenoid glucoindole alkaloids were encountered for the first time in Dipsacaceae family.
On the chemical constituents of Dipsacus asper.[Pubmed:18057739]
Chem Pharm Bull (Tokyo). 2007 Dec;55(12):1677-81.
Bioassay-guided fractionation of 95% EtOH extract from the roots of Dipsacus asper lead to the isolation of some phenolic acids (caffeic acid, 2,6-dihydroxycinnamic acid, vanillic acid, 2'-O-caffeoyl-D-glucopyranoside ester, and caffeoylquinic acid) as the major active components, and five new iridoid glucoside dimers (1-5) and one new iridoid glucoside monomer (6), other known iridoid glycosides loganin, Cantleyoside, triplostoside A, lisianthioside, 6'-O-beta-D-apiofuranosyl sweroside, as well as triterpenoids oleanic acid and akebiasaponin D. The structures of new compounds 1-6 were determined as dipsanosides C (1), D (2), E (3), F (4), G (5), and 3'-O-beta-D-glucopyranosyl sweroside (6) by spectroscopic, including 1D and 2D NMR techniques, and chemical methods.
Cantleyoside-dimethyl-acetal and other iridoid glucosides from Pterocephalus perennis--antimicrobial activities.[Pubmed:11926551]
Z Naturforsch C J Biosci. 2002 Jan-Feb;57(1-2):95-9.
Cantleyoside-dimethyl-acetal (6), was isolated from the endemic Greek plant Pterocephalus perennis subsp. perennis in addition to five other known iridoid glucosides, loganin, loganic acid, Cantleyoside, secologanin, and secologanin-dimethyl-acetal. The structure of these compounds was determined by all spectroscopic means mainly by NMR and MS techniques. The above compounds as well as their acetyl derivatives were tested against six Gram positive and negative bacteria and three pathogenic fungi.
Iridoids from Scaevola racemigera1.[Pubmed:17262339]
Planta Med. 1989 Apr;55(2):191-2.
Five iridoids have been isolated from the aerial parts of SCAEVOLA RACEMIGERA Daniker, namely, loganin, loganic acid, sylvestroside III, Cantleyoside, and scaevoloside. This latter is a novel compound whose structure 1 has been elucidated on the basis of its spectral data, mainly (1)H- and (13)C-NMR.
[Plants in New Caledonia. Iridoids from Scaevola montana Labill].[Pubmed:2637646]
Ann Pharm Fr. 1989;47(4):249-54.
Five iridoids have been isolated from the aerial parts of Scaevola montana Labill., namely loganin, sylvestroside III, sylvestroside III dimethylacetal, Cantleyoside and Cantleyoside dimethylacetal. Their structures have been elucidated on the basis of their spectral data, mainly chemical ionisation mass spectrometry and 1H-NMR spectroscopy.


