EugenitinCAS# 480-12-6 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 480-12-6 | SDF | Download SDF |
| PubChem ID | 3083581.0 | Appearance | Powder |
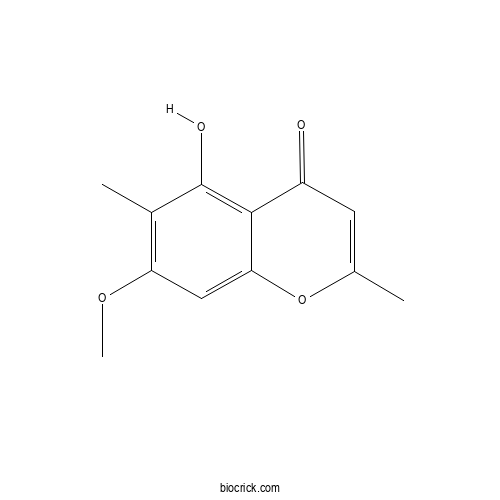
| Formula | C12H12O4 | M.Wt | 220.22 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | 5-hydroxy-7-methoxy-2,6-dimethylchromen-4-one | ||
| SMILES | CC1=CC(=O)C2=C(C(=C(C=C2O1)OC)C)O | ||
| Standard InChIKey | RGTSAUBIQAKKLC-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C12H12O4/c1-6-4-8(13)11-10(16-6)5-9(15-3)7(2)12(11)14/h4-5,14H,1-3H3 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Eugenitin Dilution Calculator

Eugenitin Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 4.5409 mL | 22.7046 mL | 45.4091 mL | 90.8183 mL | 113.5228 mL |
| 5 mM | 0.9082 mL | 4.5409 mL | 9.0818 mL | 18.1637 mL | 22.7046 mL |
| 10 mM | 0.4541 mL | 2.2705 mL | 4.5409 mL | 9.0818 mL | 11.3523 mL |
| 50 mM | 0.0908 mL | 0.4541 mL | 0.9082 mL | 1.8164 mL | 2.2705 mL |
| 100 mM | 0.0454 mL | 0.227 mL | 0.4541 mL | 0.9082 mL | 1.1352 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Ajugamacrin B
Catalog No.:BCX1354
CAS No.:123313-59-7
- Kaempferol 3-O-β-D-glucopyranosyl-(1-2)-α-L-rhamnopyranoside
Catalog No.:BCX1353
CAS No.:142451-65-8
- Umbelliprenine
Catalog No.:BCX1352
CAS No.:23838-17-7
- Yuanamide
Catalog No.:BCX1351
CAS No.:102421-42-1
- O-Methylbulbocapnine
Catalog No.:BCX1350
CAS No.:2490-83-7
- Calycanthidine
Catalog No.:BCX1349
CAS No.:5516-85-8
- Volvalerenal E
Catalog No.:BCX1348
CAS No.:1247014-33-0
- 8-Formylophiopogonone B
Catalog No.:BCX1347
CAS No.:1316224-74-4
- 5, 9-epi-Phlomiol
Catalog No.:BCX1346
CAS No.:1621908-70-0
- 9-epi-Phlomiol
Catalog No.:BCX1345
CAS No.:1621720-47-5
- 6β-Hydroxy-7-epiloganin
Catalog No.:BCX1344
CAS No.:125410-28-8
- 8-Geranyl daidzein
Catalog No.:BCX1343
CAS No.:1072940-16-9
- (3R)-dihydroarteannuin B
Catalog No.:BCX1356
CAS No.:87206-33-5
- 6""-apiosyl sec-O-glucosylhamaudol
Catalog No.:BCX1357
CAS No.:2254096-95-0
- Cantleyoside
Catalog No.:BCX1358
CAS No.:32455-46-2
- Neokurarinol
Catalog No.:BCX1359
CAS No.:52483-00-8
- Ilexoside O
Catalog No.:BCX1360
CAS No.:136552-23-3
- 6-Benzoylheteratisine
Catalog No.:BCX1361
CAS No.:99759-48-5
- Blestriarene B
Catalog No.:BCX1362
CAS No.:127211-03-4
- Gymnoside VII
Catalog No.:BCX1363
CAS No.:899430-07-0
- Crocetindial
Catalog No.:BCX1364
CAS No.:502-70-5
- Morroniaglycone
Catalog No.:BCX1365
CAS No.:1644061-02-8
- Ethyl (2E,4Z)-deca-2,4-dienoate
Catalog No.:BCX1366
CAS No.:3025-30-7
- Myristoleic acid
Catalog No.:BCX1367
CAS No.:544-64-9
Maleic anhydride and chromone derivatives from the endophytic fungus BCC 54265 (Botryosphaeriaceae).[Pubmed:29022367]
Nat Prod Res. 2018 Jul;32(13):1506-1511.
A maleic anhydride derivative, botryoanhydride (1), and a chromone derivative, botryochromone (2), together with three known chromones, Eugenitin (3), 6-hydroxymethyleugenin (4) and 6-methoxymethyleugenin (5), were isolated from cultures of the endophytic fungus BCC 54265 of the family Botryosphaeriaceae. The structures were elucidated on the basis of NMR, HRMS and CD data. Compound 2 showed weak cytotoxic activity to cancer cell-lines.
Mycoleptones A-C and polyketides from the endophyte Mycoleptodiscus indicus.[Pubmed:24387625]
J Nat Prod. 2014 Jan 24;77(1):70-8.
Three new azaphilones with an unusual methylene bridge, named mycoleptones A, B, and C (2, 4, and 5), were isolated from cultures of Mycoleptodiscus indicus, a fungus associated with the South American medicinal plant Borreria verticillata. Additionally, four known polyketides, austdiol (1), Eugenitin (3), 6-methoxyeugenin (6), and 9-hydroxyeugenin (7), were also isolated. The structural characterization of compounds was carried out by nuclear magnetic resonance spectroscopy, high-resolution mass spectrometry, electronic circular dichroism spectroscopy, time-dependent density functional theory calculations, and X-ray crystallography. Compounds 1-9 were weakly active when tested in antileishmanial and cytotoxicity assays.
Endo-xylanase GH11 activation by the fungal metabolite eugenitin.[Pubmed:22481300]
Biotechnol Lett. 2012 Aug;34(8):1487-92.
Eugenitin, a chromone derivative and a metabolite of the endophyte Mycoleptodiscus indicus, at 5 mM activated a recombinant GH11 endo-xylanase by 40 %. The in silico prediction of ligand-binding sites on the three-dimensional structure of the endo-xylanase revealed that Eugenitin interacts mainly by a hydrogen bond with a serine residue and a stacking interaction of the heterocyclic aromatic ring system with a tryptophan residue. Eugenitin improved the GH11 endo-xylanase activity on different substrates, modified the optimal pH and temperature activities and slightly affected the kinetic parameters of the enzyme.
Chemical constituents in roots of Osbeckia opipara.[Pubmed:19459302]
Zhongguo Zhong Yao Za Zhi. 2009 Feb;34(4):414-8.
OBJECTIVE: To study the chemical constituents of the roots of Osbeckia opipara. METHOD: Repeated column chromatography over silica gel, RP-18 and Sephadex LH-20, and preparative thin layer chromatography(PTLC) were used to isolate the compounds, whose structures were determined by spectroscopic methods by direct comparing spectral data with those reported references. RESULT: From the MeOH extract of the roots O. opipara, twelve compounds were isolated and identified as follows: lasiodiplodin (1) , de-O-methyllasiodiplodin (2), 2, 3- dihydro-2-hydroxy-2, 4-dimethyl-5-trans-propenylfuran-3-one (3), integracin (4), 5alpha, 8alpha-epidioxy-(22E, 24R)-ergosta-6, 22-dien-3beta-ol (5), 3, 3', 4'-tri-O-methylellagic acid (6), 5-hydroxymethyl furaldehyde (7), vomifolio (8) , betulintic acid (9), 2alpha-hydroxyursolic acid (10), (24R)-stigmast-4-ene-3-one (11), and Eugenitin (12). CONCLUSION: Compounds 1-12 were isolated from O. opipara for the first time.
Metabolites from the endophytic mitosporic Dothideomycete sp. LRUB20.[Pubmed:19038408]
Phytochemistry. 2009 Jan;70(1):121-7.
The endophytic mitosporic Dothideomycete sp. LRUB20 was found to produce pyrone derivatives, dothideopyrones A-D (1, 3, 4, and 5), together with seven known compounds, including questin (9), asterric acid (10), methyl asterrate (11), sulochrin (12), and Eugenitin (13), 6-hydroxymethylEugenitin (14), and cis, trans-muconic acid (15). Dothideopyrone D (5) and its acetate derivative 6 exhibited moderate cytotoxic activity. This is the first report on a naturally occurring muconic acid, which is commonly known as a biomarker in environments after exposure to benzene and phenol (or derivatives). Interestingly, the LRUB20 fungus could produce muconic acid in relatively high yield (47.8mg/L). The utility of endophytic fungi in the field of white biotechnology is discussed.


