DMH-1Selective BMP ALK2 receptor CAS# 1206711-16-1 |
- ML347
Catalog No.:BCC5331
CAS No.:1062368-49-3
- LDN-212854
Catalog No.:BCC5330
CAS No.:1432597-26-6
- PD 169316
Catalog No.:BCC3969
CAS No.:152121-53-4
- Imperatorin
Catalog No.:BCN5574
CAS No.:482-44-0
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 1206711-16-1 | SDF | Download SDF |
| PubChem ID | 50997747 | Appearance | Powder |
| Formula | C24H20N4O | M.Wt | 380.44 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | DMSO : 11.5 mg/mL (30.23 mM; Need ultrasonic and warming) | ||
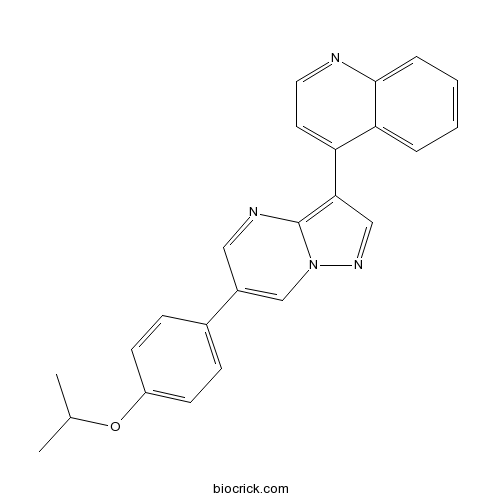
| Chemical Name | 4-[6-(4-propan-2-yloxyphenyl)pyrazolo[1,5-a]pyrimidin-3-yl]quinoline | ||
| SMILES | CC(C)OC1=CC=C(C=C1)C2=CN3C(=C(C=N3)C4=CC=NC5=CC=CC=C45)N=C2 | ||
| Standard InChIKey | JMIFGARJSWXZSH-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C24H20N4O/c1-16(2)29-19-9-7-17(8-10-19)18-13-26-24-22(14-27-28(24)15-18)20-11-12-25-23-6-4-3-5-21(20)23/h3-16H,1-2H3 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Selective inhibitor of the bone morphogenic protein (BMP) ALK2 receptor (IC50 = 108 nM). Exhibits no detectable inhibition of AMPK, ALK5, KDR (VEGFR-2) or PDGFRβ receptors. Blocks BMP4-induced phosphorylation of Smads 1, 5 and 8 in HEK293 cells. Promotes neurogenesis in human induced pluripotent stem cells (iPSCs) when used in combination with SB 431542. |

DMH-1 Dilution Calculator

DMH-1 Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.6285 mL | 13.1427 mL | 26.2854 mL | 52.5707 mL | 65.7134 mL |
| 5 mM | 0.5257 mL | 2.6285 mL | 5.2571 mL | 10.5141 mL | 13.1427 mL |
| 10 mM | 0.2629 mL | 1.3143 mL | 2.6285 mL | 5.2571 mL | 6.5713 mL |
| 50 mM | 0.0526 mL | 0.2629 mL | 0.5257 mL | 1.0514 mL | 1.3143 mL |
| 100 mM | 0.0263 mL | 0.1314 mL | 0.2629 mL | 0.5257 mL | 0.6571 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
DMH1 is a small molecule inhibitor of bone morphogenetic protein (BMP) signaling with IC50 value of 107.9nM against ALK2 [1].
DMH1 is an analog of dorsomorphin. It exclusively targets BMP and has no effect on VEGF signaling. DMH1 shows potent and specific inhibitory activity against purified human BMP type-I receptor ALK2 with IC50 value of 107.9nM. It shows no significant inhibition of purified KDR, ALK5, AMPK and PDGFRβ. In cells respectively transfected with the active forms of ALK2, ALK3 and ALK6, DMH1 effectively inhibits signaling by ALK2 and ALK3 with IC50 values of both less than 0.5μM. In addition, DMH1 has no effect on the p38/MAP kinase signaling or the Activin A-induced Smad2 activation [1].
Moreover, DMH1 is found to have antitumor effect in lung cancer through blocking BMP signaling. In the NSCLC cell line A549 cells, DMH1 inhibits the phosphorylation of Smad 1/5/8 and decreases the expression of Id1, Id2 and Id3 genes. It decreases cell migration and invasion in A549 and H460 cell line. Besides that, DMH1 reduces cell proliferation and induces cell death in A549 cells. In the A549 tumor xenograft in mice, treatment of DMH1significantly suppresses tumor growth [2].
References:
[1] Hao J, Ho J N, Lewis J A, et al. In vivo structure- activity relationship study of dorsomorphin analogues identifies selective VEGF and BMP inhibitors. ACS chemical biology, 2010, 5(2): 245-253.
[2] Hao J, Lee R, Chang A, et al. DMH1, a Small Molecule Inhibitor of BMP Type I Receptors, Suppresses Growth and Invasion of Lung Cancer. PloS one, 2014, 9(3): e90748.
- (RS)-PPG
Catalog No.:BCC6975
CAS No.:120667-15-4
- Geranyl ferulate
Catalog No.:BCN7078
CAS No.:1206615-69-1
- MK-5172 potassium salt
Catalog No.:BCC1764
CAS No.:1206524-86-8
- N-Benzylnaltrindole hydrochloride
Catalog No.:BCC6782
CAS No.:1206487-81-1
- 4,4'-Dihydroxy-3,3',9-trimethoxy-9,9'-epoxylignan
Catalog No.:BCN7017
CAS No.:1206464-65-4
- 2beta-Acetoxyferruginol
Catalog No.:BCN7955
CAS No.:1206461-56-4
- Mofegiline hydrochloride
Catalog No.:BCC5412
CAS No.:120635-25-8
- TC Mps1 12
Catalog No.:BCC7974
CAS No.:1206170-62-8
- GLPG0634
Catalog No.:BCC4145
CAS No.:1206161-97-8
- GLPG0634 analogue
Catalog No.:BCC6547
CAS No.:1206101-20-3
- 16-Nor-7,15-dioxodehydroabietic acid
Catalog No.:BCN7295
CAS No.:120591-53-9
- Anastrozole
Catalog No.:BCC4370
CAS No.:120511-73-1
- 2''-Acetylastragalin
Catalog No.:BCN4810
CAS No.:1206734-95-3
- 1-(3,4-Dihydroxyphenyl)-7-(4-hydroxyphenyl)hept-6-en-3-ol
Catalog No.:BCN7143
CAS No.:1206788-61-5
- LY2801653
Catalog No.:BCC1720
CAS No.:1206799-15-6
- LY2801653 dihydrochloride
Catalog No.:BCC1721
CAS No.:1206801-37-7
- 6-O-Nicotinoylscutebarbatine G
Catalog No.:BCN7296
CAS No.:1206805-30-2
- Bisacurone
Catalog No.:BCN6725
CAS No.:120681-81-4
- Midostaurin (PKC412)
Catalog No.:BCC5350
CAS No.:120685-11-2
- AM679
Catalog No.:BCC1354
CAS No.:1206880-66-1
- Murrayacarpin B
Catalog No.:BCN3577
CAS No.:120693-44-9
- (Z)-1-Methyl-2-(undec-6-enyl)quinolin-4(1H)-one
Catalog No.:BCN7063
CAS No.:120693-49-4
- WAY 208466 dihydrochloride
Catalog No.:BCC7807
CAS No.:1207064-61-6
- Psidial A
Catalog No.:BCN6095
CAS No.:1207181-35-8
Bone morphogenetic protein 2 increases lysyl oxidase activity via up-regulation of snail in human granulosa-lutein cells.[Pubmed:30321593]
Cell Signal. 2019 Jan;53:201-211.
Lysyl oxidase (LOX) is a copper-dependent enzyme that maintains and stabilizes the extracellular matrix (ECM) by catalyzing the cross-linking of elastin and collagen. ECM within the ovarian follicle plays a crucial role in regulating follicular development and oocyte maturation. Bone morphogenetic protein 2 (BMP2) belongs to the BMP subfamily that has been shown to be involved in the process of ovarian folliculogenesis and luteal formation. To date, whether BMP2 regulates the activity of LOX during human follicular development remains to be elucidated. The aim of this study was to investigate the effect of BMP2 on the regulation of LOX expression and activity in human granulosa-lutein cells (hGL) and the underlying mechanisms. Using both primary and immortalized (SVOG cells) hGL cells, we demonstrated that BMP2 up-regulated the expression and activity of LOX and hence decreased the soluble collagens in cultured medium in hGL cells. Additionally, the mRNA and protein levels of two transcriptional factors, SNAIL and SLUG, were increased following cell exposure to BMP2. Knockdown of SNAIL, but not SLUG partially reversed BMP2-induced increases in LOX expression and activity. The BMP2-induced up-regulation of SNAIL expression was abolished by the pre-treatment with two BMP type I receptor inhibitors, dorsomorphin and DMH-1, but not SB431542. Moreover, knockdown of SMAD4 completely abolished BMP2-induced up-regulation of SNAIL expression and the subsequent increases in LOX expression and activity. Our results suggest that BMP2 increases LOX expression and activity via the up-regulation of SNAIL in hGL cells. These findings may provide insights into the functional role of BMP2 in the regulation of ECM formation during folliculogenesis.
Bone Morphogenetic Protein 2 Promotes Human Trophoblast Cell Invasion by Inducing Activin A Production.[Pubmed:29846546]
Endocrinology. 2018 Jul 1;159(7):2815-2825.
Bone morphogenetic protein (BMP) 2 and activin A belong to the TGF-beta superfamily and are highly expressed in human endometrium and placenta. Studies have demonstrated that activin A and BMP2 play essential roles in the process of early embryo implantation by promoting human trophoblast cell invasion. However, whether activin A production can be regulated by BMP2 in human trophoblast cells remains unknown. The aim of our study was to determine the effects of BMP2 on activin A production and its role in human trophoblast invasion. Primary human extravillous trophoblast (EVT) cells were used as study models. BMP2 treatment significantly increased inhibin betaA (INHBA) mRNA levels and activin A production without altering inhibin alpha and inhibin betaB levels. BMP2-induced EVT cell invasion was attenuated by knockdown of INHBA. The increased INHBA transcription and activin A production by BMP2 were blocked by the type I receptor activin receptor (ACVR)-like kinase 2 (ALK2) and activin receptor-like kinase 3 (ALK3) inhibitor dorsomorphin homolog 1 (DMH-1). BMP2-induced INHBA upregulation was also inhibited by knockdown of type I receptor ALK3 or combined knockdown of type II receptors for BMP2 (BMPR2) and ACVR2A. Whereas BMP2 initiated both canonical SMAD1/5/8 and noncanonical SMAD2/3 signaling, only knockdown of SMAD4, but not SMAD2 and SMAD3, abolished the effects of BMP2 on INHBA. Our results show that BMP2 increases human trophoblast invasion by upregulating INHBA and activin A production via ALK3-BMPR2/ACVR2A-SMAD1/5/8-SMAD4 signaling.
SMAD1/5 mediates bone morphogenetic protein 2-induced up-regulation of BAMBI expression in human granulosa-lutein cells.[Pubmed:28578012]
Cell Signal. 2017 Sep;37:52-61.
Bone morphogenetic protein and activin membrane-bound inhibitor (BAMBI) is a transforming growth factor beta (TGF-beta) type I receptor antagonist that negatively regulates TGF-beta and bone morphogenetic protein (BMP) signaling. BAMBI has been shown to be regulated by TGF-beta signaling; however, whether BAMBI can be regulated by BMP signaling remains to be determined. The aim of this study was to investigate the effect of BMP2 on the regulation of BAMBI expression in human granulosa-lutein cells and the underlying mechanisms. Both primary and immortalized human granulosa-lutein cells were used as research models. Using dual inhibition approaches, our results showed that BMP2 activated SMAD1/5/8 phosphorylation and up-regulated BAMBI mRNA levels, which was reversed by the BMP type I receptor inhibitors, DMH-1 and dorsomorphin, but not by SB431542 (activin/TGF-beta type I receptor inhibitor). Moreover, the combined knockdown of SMAD1 and SMAD5 completely abolished the BMP2-induced up-regulation of BAMBI. Similarly, knockdown of SMAD4 reversed the BMP2-induced up-regulation of BAMBI. Pre-treatment with BMP2 inhibited the TGF-beta1-induced phosphorylation of SMAD2/3 and up-regulation of MMP2, and these inhibitory effects were reversed by knockdown of endogenous BAMBI. Our findings indicate that BAMBI is a BMP-responsive gene and that BAMBI participates in the negative feedback regulation of TGF-beta signaling in the human ovary.
Bone morphogenetic protein 2 regulates cell-cell communication by down-regulating connexin43 expression in luteinized human granulosa cells.[Pubmed:27986931]
Mol Hum Reprod. 2017 Mar 1;23(3):155-165.
STUDY QUESTION: Does bone morphogenetic protein 2 (BMP2) regulate connexin43 (Cx43) and modulate cell-cell communication in luteinized human granulosa cells? SUMMARY ANSWER: BMP2 decreases gap junction intercellular communication (GJIC) of luteinized human granulosa cells by down-regulating Cx43 expression through an activin receptor-like kinase (ALK)2/ALK3-mediated Sma- and Mad-related protein (SMAD)-dependent signaling pathway. WHAT IS KNOWN ALREADY: BMP2 and its putative receptors are highly expressed in the human corpus luteum and are involved in the process of luteolysis. Cx43-coupled gap junctions play a critical role in the development and maintenance of corpus luteum. STUDY DESIGN DURATION: This is a laboratory study conducted over a 1-year period. At least three independent experiments with three replicates were conducted and the experimental samples were compared with the appropriate vehicle controls for all of the inhibition-approach, concentration-dependent or time-course studies. PARTICIPANTS/MATERIALS, SETTING, METHODS: SVOG cell line (immortalized human granulosa-lutein cells derived from in vitro fertilization patients in an academic research center) was used as the study model. The changes of Cx43 expression and levels of phosphorylated SMAD1/5/8 protein were evaluated after exposure to recombinant human BMP2. Real-time quantitative PCR and Western blot analysis were used to examine the specific mRNA and protein levels, respectively. The BMP/TGF-beta type I receptor inhibitors (Dorsomorphin, DMH-1 and SB431542) and target depletion small interfering RNAs (ALK2, ALK3, ALK6 and SMAD4) were used to investigate the underlying molecular mechanisms. A scrape loading and dye transfer assay was used to evaluate the GJIC between the SVOG cells. MAIN RESULTS AND THE ROLE OF CHANCE: Treatment with BMP2 down-regulated the expression of Cx43 and decreased the GJIC activity, whereas it increased the phosphorylated SMAD1/5/8 protein in SVOG cells (P < 0.05). These biological effects were abolished by pre-treatment with the BMP type I receptor inhibitors, Dorsomorphin and DMH-1 (P < 0.05), but not SB431542. Additionally, the individual or concomitant small interfering RNA-mediated knockdown of ALK2 and ALK3, but not ALK6 attenuated the BMP2-induced increases in phosphorylated SMAD1/5/8 and down-regulation of Cx43 expression (P < 0.05). The knockdown of SMAD4 completely abolished the BMP2-induced down-regulation of Cx43 expression (P < 0.05). LIMITATIONS REASONS FOR CAUTION: This experimental study was conducted in an in vitro cell culture system, and may not reflect a realistic intra-ovarian environment. WIDER IMPLICATIONS OF THE FINDINGS: Our results suggested that BMP2 may be involved in the local modulation of cell-cell communication in the luteal phase. This study also represents the first comprehensive research of molecular mechanisms of BMP2 in the down-regulation Cx43 in luteinized human granulosa cells. Such data may provide valuable insights into ovarian physiology and benefit the development of potential therapeutic methods for patients suffering from luteal insufficiency. LARGE SCALE DATA: N/A. STUDY FUNDING AND COMPETING INTEREST(s): This research was supported by an operating grant from the China-Canadian Joint Health Research Initiative Grants Program to P.C.K. Leung and J.Z. Sheng. The authors declare no competing interest with the contents of this article.
Recombinant BMP4 and BMP7 downregulate pentraxin 3 in human granulosa cells.[Pubmed:25514099]
J Clin Endocrinol Metab. 2015 Mar;100(3):E365-74.
CONTEXT: Theca cell-derived bone morphogenetic protein 4 (BMP4) and BMP7 are important regulators of folliculogenesis and have been shown to inhibit luteinization. Pentraxin 3 (PTX3) plays a critical role in the assembly of the cumulus oophorus extracellular matrix, which is essential for cumulus expansion during ovulation and may be modulated by BMP4 and BMP7. OBJECTIVE: The aim of this study was to investigate the effects of BMP4 and BMP7 on the expression of PTX3 in human granulosa cells and to examine their underlying molecular determinants. DESIGN: An established immortalized human granulosa cell line (SVOG), a granulosa cell tumor cell line (KGN), and primary granulosa-lutein cells were used as study models. PTX3 expression and accumulation as well as Smad1/5/8 phosphorylation were examined after exposure to recombinant human BMP4 and BMP7. BMP type I receptor involvement was investigated with inhibitors (dorsomorphin and DMH-1 (4-[6-[4-(1-Methylethoxy)phenyl]pyrazolo[1,5-a]pyrimidin-3-yl]-quinoline)) and small interfering RNAs targeting activin receptor-like kinase (ALK)2, ALK3, and/or ALK6. Small interfering RNAs targeting Smad4 were used to verify the involvement of Smad signaling. SETTING: The study was conducted at an academic research center. MAIN OUTCOME MEASURES: Quantitative RT-PCR and Western blot were used to measure mRNA and protein levels, respectively. Levels of PTX3 and BMP4 were measured by ELISA. RESULTS: Treatment with BMP4 and BMP7 significantly decreased PTX3 mRNA and protein production. These suppressive effects, along with the induction of Smad1/5/8 phosphorylation, were attenuated by cotreatment with 2 BMP type I receptor inhibitors (dorsomorphin and/or DMH-1). Combined knockdown (ALK3/ALK6 for BMP4 and ALK2/ALK3 for BMP7) reversed the effects of BMP4- and BMP7-induced Smad1/5/8 phosphorylation and PTX3 suppression. Furthermore, Smad4 knockdown reversed the suppressive effects of BMP4 and BMP7 on PTX3 expression. In follicular fluid, concentrations of PTX3 were negatively correlated with concentrations of BMP4. CONCLUSION: BMP4 and BMP7 use differential subsets of BMP type I receptors to downregulate PTX3 expression via Smad-dependent signaling in human granulosa cells.
BMP15 suppresses progesterone production by down-regulating StAR via ALK3 in human granulosa cells.[Pubmed:24140593]
Mol Endocrinol. 2013 Dec;27(12):2093-104.
In addition to somatic cell-derived growth factors, oocyte-derived growth differentiation factor (GDF)9 and bone morphogenetic protein (BMP)15 play essential roles in female fertility. However, few studies have investigated their effects on human ovarian steroidogenesis, and fewer still have examined their differential effects or underlying molecular determinants. In the present study, we used immortalized human granulosa cells (SVOG) and human granulosa cell tumor cells (KGN) to compare the effects of GDF9 and BMP15 on steroidogenic enzyme expression and investigate potential mechanisms of action. In SVOG cells, neither GDF9 nor BMP15 affects the mRNA levels of P450 side-chain cleavage enzyme or 3beta-hydroxysteroid dehydrogenase. However, treatment with BMP15, but not GDF9, significantly decreases steroidogenic acute regulatory protein (StAR) mRNA and protein levels as well as progesterone production. These suppressive effects, along with the induction of Sma and Mad-related protein (SMAD)1/5/8 phosphorylation, are attenuated by cotreatment with 2 different BMP type I receptor inhibitors (dorsomorphin and DMH-1). Furthermore, depletion of activin receptor-like kinase (ALK)3 using small interfering RNA reverses the effects of BMP15 on SMAD1/5/8 phosphorylation and StAR expression. Similarly, knockdown of ALK3 abolishes BMP15-induced SMAD1/5/8 phosphorylation in KGN cells. These results provide evidence that oocyte-derived BMP15 down-regulates StAR expression and decreases progesterone production in human granulosa cells, likely via ALK3-mediated SMAD1/5/8 signaling. Our findings suggest that oocyte may play a critical role in the regulation of progesterone to prevent premature luteinization during the late stage of follicle development.
(+)-Cannabidiol analogues which bind cannabinoid receptors but exert peripheral activity only.[Pubmed:15588739]
Eur J Pharmacol. 2004 Dec 15;506(2):179-88.
Delta9-Tetrahydrocannabinol (Delta9-THC) and (-)-cannabidiol are major constituents of the Cannabis sativa plant with different pharmacological profiles: (-)-Delta9-tetrahydrocannabinol, but not (-)-cannabidiol, activates cannabinoid CB1 and CB2 receptors and induces psychoactive and peripheral effects. We have tested a series of (+)-cannabidiol derivatives, namely, (+)-cannabidiol-DMH (DMH-1,1-dimethylheptyl-), (+)-7-OH-cannabidiol-DMH, (+)-7-OH- cannabidiol, (+)-7-COOH- cannabidiol and (+)-7-COOH-cannabidiol-DMH, for central and peripheral (intestinal, antiinflammatory and peripheral pain) effects in mice. Although all (+)-cannabidiols bind to cannabinoid CB1 and CB2 receptors, only (+)-7-OH-cannabidiol-DMH was centrally active, while all (+)-cannabidiol analogues completely arrested defecation. The effects of (+)-cannabidiol-DMH and (+)-7-OH-cannabidiol-DMH were partially antagonized by the cannabinoid CB1 receptor antagonist N-(piperidiny-1-yl)-5-(4-chlorophenyl)-1-(2,4-dichlorophenyl)-4-methyl-1H-pyrazol e-3-carboxamide (SR141716), but not by the cannabinoid CB2 receptor antagonist N-[-(1S)-endo-1,3,3-trimethil bicyclo [2.2.1] heptan-2-yl-5-(4-chloro-3-methylphenyl)-1-(4-methylbenzyl)-pyrazole-3-carboxamide (SR144528), and had no effect on CB1(-/-) receptor knockout mice. (+)-Cannabidiol-DMH inhibited the peripheral pain response and arachidonic-acid-induced inflammation of the ear. We conclude that centrally inactive (+)-cannabidiol analogues should be further developed as antidiarrheal, antiinflammatory and analgesic drugs for gastrointestinal and other peripheral conditions.
DMH1, a highly selective small molecule BMP inhibitor promotes neurogenesis of hiPSCs: comparison of PAX6 and SOX1 expression during neural induction.[Pubmed:22860217]
ACS Chem Neurosci. 2012 Jun 20;3(6):482-91.
Recent successes in deriving human-induced pluripotent stem cells (hiPSCs) allow for the possibility of studying human neurons derived from patients with neurological diseases. Concomitant inhibition of the BMP and TGF-beta1 branches of the TGF-beta signaling pathways by the endogenous antagonist, Noggin, and the small molecule SB431542, respectively, induces efficient neuralization of hiPSCs, a method known as dual-SMAD inhibition. The use of small molecule inhibitors instead of their endogenous counterparts has several advantages including lower cost, consistent activity, and the maintenance of xeno-free culture conditions. We tested the efficacy of DMH1, a highly selective small molecule BMP-inhibitor for its potential to replace Noggin in the neuralization of hiPSCs. We compare Noggin and DMH1-induced neuralization of hiPSCs by measuring protein and mRNA levels of pluripotency and neural precursor markers over a period of seven days. The regulation of five of the six markers assessed was indistinguishable in the presence of concentrations of Noggin or DMH1 that have been shown to effectively inhibit BMP signaling in other systems. We observed that by varying the DMH1 or Noggin concentration, we could selectively modulate the number of SOX1 expressing cells, whereas PAX6, another neural precursor marker, remained the same. The level and timing of SOX1 expression have been shown to affect neural induction as well as neural lineage. Our observations, therefore, suggest that BMP-inhibitor concentrations need to be carefully monitored to ensure appropriate expression levels of all transcription factors necessary for the induction of a particular neuronal lineage. We further demonstrate that DMH1-induced neural progenitors can be differentiated into beta3-tubulin expressing neurons, a subset of which also express tyrosine hydroxylase. Thus, the combined use of DMH1, a highly specific BMP-pathway inhibitor, and SB431542, a TGF-beta1-pathway specific inhibitor, provides us with the tools to independently regulate these two pathways through the exclusive use of small molecule inhibitors.
Swimming into the future of drug discovery: in vivo chemical screens in zebrafish.[Pubmed:20166761]
ACS Chem Biol. 2010 Feb 19;5(2):159-61.
In recent years in vivo chemical screening in zebrafish has emerged as a rapid and efficient method to identify lead compounds that modulate specific biological processes. By performing primary screening in vivo, the bioactivity, toxicity, and off-target side effects are determined from the onset of drug development. A recent study demonstrates that in vivo screening can be used successfully to perform structure-activity relationship (SAR) studies. This work validates the zebrafish as an effective model for not only drug discovery but also drug optimization.
In vivo structure-activity relationship study of dorsomorphin analogues identifies selective VEGF and BMP inhibitors.[Pubmed:20020776]
ACS Chem Biol. 2010 Feb 19;5(2):245-53.
The therapeutic potential of small molecule signaling inhibitors is often limited by off-target effects. Recently, in a screen for compounds that perturb the zebrafish embryonic dorsoventral axis, we identified dorsomorphin, the first selective inhibitor of bone morphogenetic protein (BMP) signaling. Here we show that dorsomorphin has significant "off-target" effects against the VEGF (vascular endothelial growth factor) type-2 receptor (Flk1/KDR) and disrupts zebrafish angiogenesis. Since both BMP and VEGF signals are known to be involved in vascular development, we sought to determine whether dorsomorphin's antiangiogenic effects are due to its impact on the BMP or VEGF signals through the development of analogues that target BMP but not VEGF signaling and vice versa. In a structure-activity relationship (SAR) study of dorsomorphin analogues based primarily on their effects on live zebrafish embryos, we identified highly selective and potent BMP inhibitors as well as selective VEGF inhibitors. One of the BMP inhibitors, DMH1, which exclusively targets the BMP but not the VEGF pathway, dorsalized the embryonic axis without disrupting the angiogenic process, demonstrating that BMP signaling was not involved in the angiogenic process. This is one of the first full-scale SAR studies performed in vertebrates and demonstrates the potential of zebrafish as an attractive complementary platform for drug development that incorporates an assessment of in vivo bioactivity and selectivity in the context of a living organism.


