AM679FLAP inhibitor CAS# 1206880-66-1 |
- BAY-X 1005
Catalog No.:BCC6038
CAS No.:128253-31-6
- MK-0591
Catalog No.:BCC1753
CAS No.:136668-42-3
- MK591
Catalog No.:BCC1766
CAS No.:147030-01-1
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 1206880-66-1 | SDF | Download SDF |
| PubChem ID | 44627267 | Appearance | Powder |
| Formula | C40H44N4O5S | M.Wt | 692.87 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | DMSO : ≥ 100 mg/mL (144.33 mM) H2O : < 0.1 mg/mL (insoluble) *"≥" means soluble, but saturation unknown. | ||
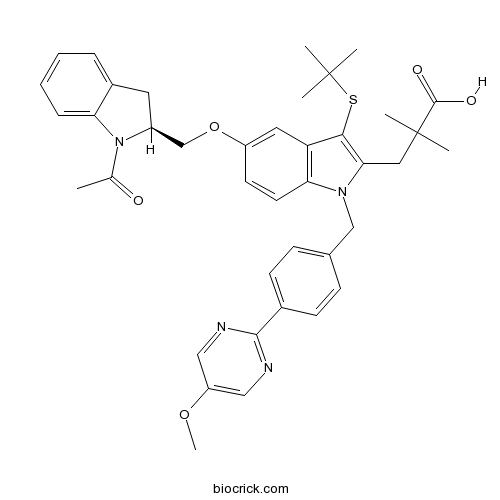
| Chemical Name | 3-[5-[[(2S)-1-acetyl-2,3-dihydroindol-2-yl]methoxy]-3-tert-butylsulfanyl-1-[[4-(5-methoxypyrimidin-2-yl)phenyl]methyl]indol-2-yl]-2,2-dimethylpropanoic acid | ||
| SMILES | CC(=O)N1C(CC2=CC=CC=C21)COC3=CC4=C(C=C3)N(C(=C4SC(C)(C)C)CC(C)(C)C(=O)O)CC5=CC=C(C=C5)C6=NC=C(C=N6)OC | ||
| Standard InChIKey | VYXWHVDEWWHDLH-LJAQVGFWSA-N | ||
| Standard InChI | InChI=1S/C40H44N4O5S/c1-25(45)44-29(18-28-10-8-9-11-33(28)44)24-49-30-16-17-34-32(19-30)36(50-39(2,3)4)35(20-40(5,6)38(46)47)43(34)23-26-12-14-27(15-13-26)37-41-21-31(48-7)22-42-37/h8-17,19,21-22,29H,18,20,23-24H2,1-7H3,(H,46,47)/t29-/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | AM679 is a selective inhibitor of 5-Lipoxygenase-activating protein (FLAP). | |||||
| Targets | FLAP | |||||

AM679 Dilution Calculator

AM679 Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 1.4433 mL | 7.2164 mL | 14.4327 mL | 28.8654 mL | 36.0818 mL |
| 5 mM | 0.2887 mL | 1.4433 mL | 2.8865 mL | 5.7731 mL | 7.2164 mL |
| 10 mM | 0.1443 mL | 0.7216 mL | 1.4433 mL | 2.8865 mL | 3.6082 mL |
| 50 mM | 0.0289 mL | 0.1443 mL | 0.2887 mL | 0.5773 mL | 0.7216 mL |
| 100 mM | 0.0144 mL | 0.0722 mL | 0.1443 mL | 0.2887 mL | 0.3608 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
AM679 is a topically applied and potent 5-lipoxygenase-activating protein (FLAP) inhibitor with an IC50 value of 2.2 nM [1] [2].
FLAP and 5-lipoxygenase (5-LO) together convert membrane-derived arachidonic acid to the pro-inflammatory mediator leukotriene epoxide LTA4. LTA4 is hence rapidly converted into either LTB4 by LTA4 hydrolase or LTC4 by LTC4 synthase [2].
Incubation with AM679 for an extended time period (5 h) increased the potency of human blood against LTB4 production with an IC50 value of 53 nM. This time-dependent increase also happened with an IC50 value of 9 nM in rat blood incubated with AM679 for 4 h [2].
Cysteinyl leukotrienes (CysLTs) are known as promoters of inflammation and allergy. Mouse eye infected by RSV began to show increased ocular CysLTs 4 days after infection. AM679 decreased the peak 6- to 8-day of ocular CysLTs by more than 90%. By day 10, concentrations of CysLT for both AM679-treated mouse eyes and control had almost returned to the baseline. A strong correlation between RSV and IL-4 mRNA had been found for human allergic conjunctivitis. 6 days after RSV infection, IL-4 mRNA concentrations were significantly elevated in RSV-infected mouse eyes. Around 14 days, IL-4 mRNA concentrations were hence gradually decreased to near baseline. AM679 could inhibit more than 80% of the IL-4 increase resulted from RSV infection [1].
References:
[1]. Alla Musiyenko, Lucia Correa, Nicholas Stock, et al. A Novel 5-Lipoxygenase-Activating Protein Inhibitor, AM679, Reduces Inflammation in the Respiratory Syncytial Virus-Infected Mouse Eye. Clinical and Vaccine Immunology, 2009, 16(11):1654-1659.
[2]. Nicholas Stock, Christopher Baccei, Gretchen Bain, et al. 5-Lipoxygenase-activating protein inhibitors. Part 2: 3-{5-((S)-1-Acetyl-2,3-dihydro-
1H-indol-2-ylmethoxy)-3-tert-butylsulfanyl-1-[4-(5-methoxy-pyrimidin-2-yl)-benzyl]-1H-indol-2-yl}-2,2-dimethyl-propionic acid (AM679)—A potent FLAP inhibitor. Bioorganic & Medicinal Chemistry Letters, 2010, 20:213-217.
- Midostaurin (PKC412)
Catalog No.:BCC5350
CAS No.:120685-11-2
- Bisacurone
Catalog No.:BCN6725
CAS No.:120681-81-4
- 6-O-Nicotinoylscutebarbatine G
Catalog No.:BCN7296
CAS No.:1206805-30-2
- LY2801653 dihydrochloride
Catalog No.:BCC1721
CAS No.:1206801-37-7
- LY2801653
Catalog No.:BCC1720
CAS No.:1206799-15-6
- 1-(3,4-Dihydroxyphenyl)-7-(4-hydroxyphenyl)hept-6-en-3-ol
Catalog No.:BCN7143
CAS No.:1206788-61-5
- 2''-Acetylastragalin
Catalog No.:BCN4810
CAS No.:1206734-95-3
- DMH-1
Catalog No.:BCC5329
CAS No.:1206711-16-1
- (RS)-PPG
Catalog No.:BCC6975
CAS No.:120667-15-4
- Geranyl ferulate
Catalog No.:BCN7078
CAS No.:1206615-69-1
- MK-5172 potassium salt
Catalog No.:BCC1764
CAS No.:1206524-86-8
- N-Benzylnaltrindole hydrochloride
Catalog No.:BCC6782
CAS No.:1206487-81-1
- Murrayacarpin B
Catalog No.:BCN3577
CAS No.:120693-44-9
- (Z)-1-Methyl-2-(undec-6-enyl)quinolin-4(1H)-one
Catalog No.:BCN7063
CAS No.:120693-49-4
- WAY 208466 dihydrochloride
Catalog No.:BCC7807
CAS No.:1207064-61-6
- Psidial A
Catalog No.:BCN6095
CAS No.:1207181-35-8
- Scutebata A
Catalog No.:BCN6096
CAS No.:1207181-57-4
- Scutebata B
Catalog No.:BCN6097
CAS No.:1207181-58-5
- Scutebata C
Catalog No.:BCN6098
CAS No.:1207181-59-6
- Scutebata E
Catalog No.:BCN6099
CAS No.:1207181-61-0
- Scutebata F
Catalog No.:BCN6100
CAS No.:1207181-62-1
- Scutebata G
Catalog No.:BCN6101
CAS No.:1207181-63-2
- Sarcandrolide D
Catalog No.:BCN6621
CAS No.:1207185-03-2
- 12alpha-Hydroxyevodol
Catalog No.:BCN6102
CAS No.:120722-04-5
5-Lipoxygenase-activating protein inhibitors. Part 2: 3-{5-((S)-1-Acetyl-2,3-dihydro-1H-indol-2-ylmethoxy)-3-tert-butylsulfanyl-1-[4-(5 -methoxy-pyrimidin-2-yl)-benzyl]-1H-indol-2-yl}-2,2-dimethyl-propionic acid (AM679)--a potent FLAP inhibitor.[Pubmed:19914828]
Bioorg Med Chem Lett. 2010 Jan 1;20(1):213-7.
A series of potent 5-lipoxygenase-activating protein (FLAP) inhibitors are herein described. SAR studies focused on the discovery of novel alicyclic moieties appended to an indole core to optimize potency, physical properties and off-target activities. Subsequent SAR on the N-benzyl substituent of the indole led to the discovery of compound 39 (AM679) which showed potent inhibition of leukotrienes in human blood and in a rodent bronchoalvelolar lavage (BAL) challenge model.
Identification of two cannabimimetic compounds WIN48098 and AM679 in illegal products.[Pubmed:23937936]
Sci Justice. 2013 Sep;53(3):286-92.
Two synthetic cannabinoids have been identified, during a survey, as new adulterants; they might have been intended to be used as ingredients for smart drugs. The characterization of these compounds has been made by gas chromatography-mass spectrometry (GC-MS), Orbitrap high resolution mass spectrometry (HRMS) and nuclear magnetic resonance (NMR), leading to the identification of WIN48098, a compound disclosed as a new adulterant in herbal and powder products, and AM679, identified in Italy for the first time. Taking into account the high number of synthetic cannabinoids seized during the last year in Italy, how quickly they appear on the illegal market and the rapidity required for analytical results, a method was developed for the simultaneous quantitation of several synthetic cannabinoids, using gas chromatography-flame ionization detection (GC-FID).
A novel 5-lipoxygenase-activating protein inhibitor, AM679, reduces inflammation in the respiratory syncytial virus-infected mouse eye.[Pubmed:19759251]
Clin Vaccine Immunol. 2009 Nov;16(11):1654-9.
Respiratory syncytial virus (RSV) is an important cause of viral respiratory disease in children, and RSV bronchiolitis has been associated with the development of asthma in childhood. RSV spreads from the eye and nose to the human respiratory tract. Correlative studies of humans and direct infection studies of BALB/c mice have established the eye as a significant pathway of entry of RSV to the lung. At the same time, RSV infection of the eye produces symptoms resembling allergic conjunctivitis. Cysteinyl leukotrienes (CysLTs) are known promoters of allergy and inflammation, and the first step in their biogenesis from arachidonic acid is catalyzed by 5-lipoxygenase (5-LO) in concert with the 5-LO-activating protein (FLAP). We have recently developed a novel compound, AM679, which is a topically applied and potent inhibitor of FLAP. Here we show with the BALB/c mouse eye RSV infection model that AM679 markedly reduced the RSV-driven ocular pathology as well as the synthesis of CysLTs in the eye. In addition, AM679 decreased the production of the Th2 cell cytokine interleukin-4 but did not increase the viral load in the eye or the lung. These results suggest that FLAP inhibitors may be therapeutic for RSV-driven eye disease and possibly other inflammatory eye indications.


