DulcitolCAS# 608-66-2 |
- Allitol
Catalog No.:BCN5593
CAS No.:488-44-8
- D-Mannitol
Catalog No.:BCN2205
CAS No.:69-65-8
- Sorbitol
Catalog No.:BCX0672
CAS No.:50-70-4
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 608-66-2 | SDF | Download SDF |
| PubChem ID | 11850 | Appearance | Powder |
| Formula | C6H14O6 | M.Wt | 182.07 |
| Type of Compound | Miscellaneous | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
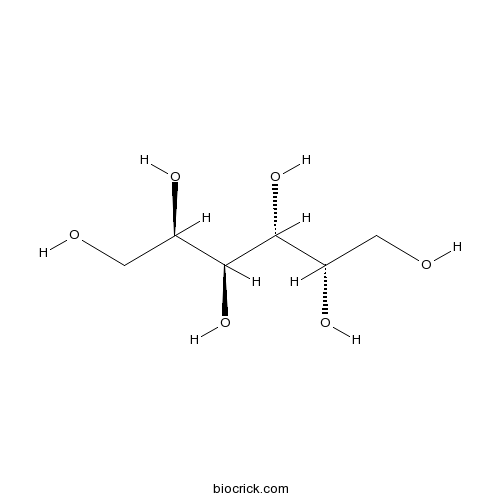
| Chemical Name | (2R,3S,4R,5S)-hexane-1,2,3,4,5,6-hexol | ||
| SMILES | C(C(C(C(C(CO)O)O)O)O)O | ||
| Standard InChIKey | FBPFZTCFMRRESA-GUCUJZIJSA-N | ||
| Standard InChI | InChI=1S/C6H14O6/c7-1-3(9)5(11)6(12)4(10)2-8/h3-12H,1-2H2/t3-,4+,5+,6- | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | 1. Dulcitol has antitumor activity. |

Dulcitol Dilution Calculator

Dulcitol Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 5.4924 mL | 27.462 mL | 54.9239 mL | 109.8479 mL | 137.3098 mL |
| 5 mM | 1.0985 mL | 5.4924 mL | 10.9848 mL | 21.9696 mL | 27.462 mL |
| 10 mM | 0.5492 mL | 2.7462 mL | 5.4924 mL | 10.9848 mL | 13.731 mL |
| 50 mM | 0.1098 mL | 0.5492 mL | 1.0985 mL | 2.197 mL | 2.7462 mL |
| 100 mM | 0.0549 mL | 0.2746 mL | 0.5492 mL | 1.0985 mL | 1.3731 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- 5,7,8-Trimethoxycoumarin
Catalog No.:BCN4125
CAS No.:60796-65-8
- Norbraylin
Catalog No.:BCN4124
CAS No.:60796-64-7
- Berbamine hydrochloride
Catalog No.:BCN2400
CAS No.:6078-17-7
- Quercetin 3-O-beta-D-glucose-7-O-beta-D-gentiobioside
Catalog No.:BCN7821
CAS No.:60778-02-1
- SB742457
Catalog No.:BCC5058
CAS No.:607742-69-8
- A 77-01
Catalog No.:BCC1318
CAS No.:607737-87-1
- Ethyl 4-methoxysalicylate
Catalog No.:BCN3499
CAS No.:35031-00-6
- Meloside A
Catalog No.:BCN2278
CAS No.:60767-80-8
- ent-Kauran-17,19-dioic acid
Catalog No.:BCN4122
CAS No.:60761-79-7
- 2-Hydroxy-1,8-cineole
Catalog No.:BCN4121
CAS No.:60761-00-4
- Canthin-6-one N-oxide
Catalog No.:BCN2992
CAS No.:60755-87-5
- AZ 10606120 dihydrochloride
Catalog No.:BCC6005
CAS No.:607378-18-7
- Sinomenine HCl
Catalog No.:BCN6318
CAS No.:6080-33-7
- Lithium Citrate
Catalog No.:BCC3804
CAS No.:6080-58-6
- 4-Methoxy-1-methoxycarbonyl-beta-carboline
Catalog No.:BCN1401
CAS No.:60807-25-2
- Apremilast (CC-10004)
Catalog No.:BCC2273
CAS No.:608141-41-9
- Esculentoside B
Catalog No.:BCN5011
CAS No.:60820-94-2
- Crotaverrine
Catalog No.:BCN2142
CAS No.:60827-69-2
- Ligularidine
Catalog No.:BCN2141
CAS No.:60872-63-1
- PIR 3.5
Catalog No.:BCC6128
CAS No.:6088-51-3
- H-DL-Pro-OH
Catalog No.:BCC3026
CAS No.:609-36-9
- 6-Hydroxycoumarin
Catalog No.:BCC9207
CAS No.:6093-68-1
- YZ9
Catalog No.:BCC8001
CAS No.:6093-71-6
- Bz-Glu-OH
Catalog No.:BCC2922
CAS No.:6094-36-6
Osmotic stress activates nif and fix genes and induces the Rhizobium tropici CIAT 899 Nod factor production via NodD2 by up-regulation of the nodA2 operon and the nodA3 gene.[Pubmed:30917160]
PLoS One. 2019 Mar 27;14(3):e0213298.
The symbiosis between rhizobia and legumes is characterized by a complex molecular dialogue in which the bacterial NodD protein plays a major role due to its capacity to activate the expression of the nodulation genes in the presence of appropiate flavonoids. These genes are involved in the synthesis of molecules, the nodulation factors (NF), responsible for launching the nodulation process. Rhizobium tropici CIAT 899, a rhizobial strain that nodulates Phaseolus vulgaris, is characterized by its tolerance to multiple environmental stresses such as high temperatures, acidity or elevated osmolarity. This strain produces nodulation factors under saline stress and the same set of CIAT 899 nodulation genes activated by inducing flavonoids are also up-regulated in a process controlled by the NodD2 protein. In this paper, we have studied the effect of osmotic stress (high mannitol concentrations) on the R. tropici CIAT 899 transcriptomic response. In the same manner as with saline stress, the osmotic stress mediated NF production and export was controlled directly by NodD2. In contrast to previous reports, the nodA2FE operon and the nodA3 and nodD1 genes were up-regulated with mannitol, which correlated with an increase in the production of biologically active NF. Interestingly, in these conditions, this regulatory protein controlled not only the expression of nodulation genes but also the expression of other genes involved in protein folding and synthesis, motility, synthesis of polysaccharides and, surprinsingly, nitrogen fixation. Moreover, the non-metabolizable sugar Dulcitol was also able to induce the NF production and the activation of nod genes in CIAT 899.
Development and validation of HILIC-UHPLC-ELSD methods for determination of sugar alcohols stereoisomers and its application for bioconversion processes of crude glycerin.[Pubmed:30621908]
J Chromatogr A. 2019 Mar 29;1589:56-64.
The recent increase in the production of crude glycerin through the manufacture of biodiesel has imputed a commercial issue, the excess of this raw material in the market and its constant devaluation, which resulted in the need for new technologies for its use. Crude glycerin can be used in biotechnological processes for the production of high value-added compounds. This study presents novel, simple and fast methods based on ultra-high performance liquid chromatography (UHPLC) using evaporative light scattering detection (ELSD) for simultaneous analysis of ten sugar alcohols with a hydrophilic interaction chromatography (HILIC) column. The selected compounds and their possible stereoisomers have major commercial importance and they can be obtained by biotechnological routes. Under optimized conditions, threitol, erythritol, adonitol, xylitol, arabitol, iditol, sorbitol, mannitol, Dulcitol and volemitol can be analyzed simultaneously within 15.0 min. The use of different column temperatures was a key parameter to reach the selectivity during the separation of some stereoisomers. Regression equations revealed a good linear relationship (R > 0.995) over the range from 50.0 to 800.0 ng. Limits of detection (LOD) and quantification (LOQ) ranged from 30.0 to 45.0 ng and 50.0-75.0 ng, respectively. The HILIC-UHPLC-ELSD methods showed good precision with low coefficient of variation (CV%) for the intra- and inter-assays experiments (
Sensitivity to antimicrobials of faecal Buttiauxella spp. from roe and red deer (Capreolus capreolus, Cervus elaphus)detected with MALDI-TOF mass spectrometry.[Pubmed:30468327]
Pol J Vet Sci. 2018 Sep;21(3):543-547.
Wild ruminants are an interesting topic for research because only limited information exists regarding their microbiota. They could also be an environmental reservoir of undesirable bacteria for other animals or humans. In this study faeces of the 21 free-living animals was sampled (9 Cervus elaphus-red deer, adult females, 12 Capreolus capreolus-roe deer, young females). They were culled by selective-reductive shooting during the winter season of 2014/2015 in the Strzalowo Forest District-Piska Primeval Forest (53 degrees 36 min 43.56 sec N, 21 degrees 30 min 58.68 sec E) in Poland. Buttiauxella sp. is a psychrotolerant, facultatively anaerobic, Gram-negative rod anaerobic bacte- rial species belonging to the Phylum Proteobacteria, Class Gammaproteobacteria, Order Entero- bacteriales, Family Enterobacteriacae and to Genus Buttiauxella. Buttiauxella sp. has never previ- ously been reported in wild ruminants. In this study, identification, antimicrobial profile and sensitivity to enterocins of Buttiauxella strains were studied as a contribution to the microbiota of wild animals, but also to extend knowledge regarding the antimicrobial spectrum of enterocins. Five strains were identified using the MALDI-TOF identification system (evaluation score value was up to 2.224) and allotted to the genus Buttiauxella including the species Buttiauxella gaviniae, B. ferragutiae, B. agrestis. Strains were DNase negative, and they hydrolysed esculin; fermentation of L-arabinose, D-mannitol and D-mannose was positive. Dulcitol, inositol reaction, urea and indol were negative. Buttiauxella strains did not form biofilm. They were resistant to at least one of the 13 antibiotics tested. B. agrestis 2/109/1 was resistant to amdinocillin, clindamycin and pen- icillin. However, Buttiauxella strains were sensitive to the enterocins used (inhibition activity ranged from 100 to 25 600 AU/ml).
Inhibitory effects of Dulcitol on rat C6 glioma by regulating autophagy pathway.[Pubmed:30445865]
Nat Prod Res. 2018 Nov 16:1-5.
In the study, we treated C6 rat glioma cells with 25 mg/ml Dulcitol for 24 h. 3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assays were used to detect cellular growth. The measurements of the superoxide dismutase (SOD), malondialdehyde (MDA) and catalase (CAT) were used to assess oxidative stress level. Western was performed to detect the autophagy and apoptosis expression. The data showed that Dulcitol significantly decreased the cell viability, upregulated the Bax level in mitochondria and the Cytochrome C level in cytoplasm, and downregulated anti-apoptotic protein Bcl-xl. Moreover, it enhanced MDA level, reduced CAT and SOD activities, decreased LC3-II/LC3-I ratio, and increased P62 expression. However, rapamycin increased autophagy level and cell viability, and decreased ROS in Dulcitol treated C6 cells. Moreover, Dulcitol inhibited the glioma growth and enhanced survival in vivo. These results suggest that Dulcitol evidently increase cellular ROS levels and apoptosis in glioma cells, which can be significantly regulated by autophagy.


