Apremilast (CC-10004)PDE4 inhibitor CAS# 608141-41-9 |
- Cilomilast
Catalog No.:BCC2283
CAS No.:153259-65-5
- CDP 840 hydrochloride
Catalog No.:BCC7814
CAS No.:162542-90-7
- Rolipram
Catalog No.:BCC2282
CAS No.:61413-54-5
- Oglemilast
Catalog No.:BCC1817
CAS No.:778576-62-8
- GSK256066
Catalog No.:BCC2285
CAS No.:801312-28-7
- AN-2728
Catalog No.:BCC1361
CAS No.:906673-24-3
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 608141-41-9 | SDF | Download SDF |
| PubChem ID | 11561674 | Appearance | Powder |
| Formula | C22H24N2O7S | M.Wt | 460.5 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | CC-10004 | ||
| Solubility | DMSO : ≥ 50 mg/mL (108.58 mM) H2O : < 0.1 mg/mL (insoluble) *"≥" means soluble, but saturation unknown. | ||
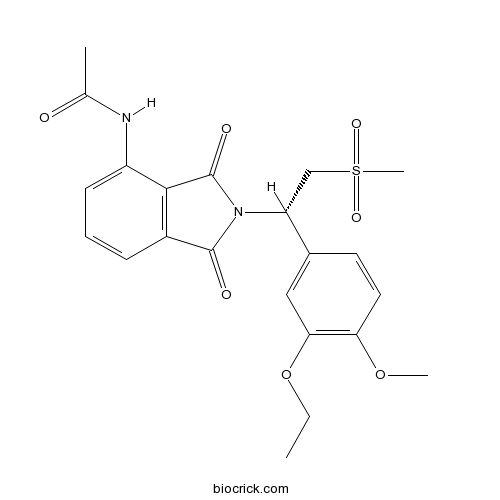
| Chemical Name | N-[2-[(1S)-1-(3-ethoxy-4-methoxyphenyl)-2-methylsulfonylethyl]-1,3-dioxoisoindol-4-yl]acetamide | ||
| SMILES | CCOC1=C(C=CC(=C1)C(CS(=O)(=O)C)N2C(=O)C3=C(C2=O)C(=CC=C3)NC(=O)C)OC | ||
| Standard InChIKey | IMOZEMNVLZVGJZ-QGZVFWFLSA-N | ||
| Standard InChI | InChI=1S/C22H24N2O7S/c1-5-31-19-11-14(9-10-18(19)30-3)17(12-32(4,28)29)24-21(26)15-7-6-8-16(23-13(2)25)20(15)22(24)27/h6-11,17H,5,12H2,1-4H3,(H,23,25)/t17-/m1/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Apremilast(CC10004) is a novel small-molecule inhibitor of PDE4 with an IC50 value of 74 nM. | |||||
| Targets | PDE4 | |||||
| IC50 | 74 nM | |||||
| Cell experiment [1]: | |
| Cell lines | U937 human monocytic cells |
| Preparation method | The solubility of this compound in DMSO is >10 mM. General tips for obtaining a higher concentration: Please warm the tube at 37 °C for 10 minutes and/or shake it in the ultrasonic bath for a while.Stock solution can be stored below -20°C for several months. |
| Reacting condition | 18h; IC50=74 nM |
| Applications | Apremilast was initially screened for PDE4 inhibition using a partially purified enzyme preparation from U937 human monocytic cells and which has been shown previously to contain predominantly PDE4B and PDE4D activities. Apremilast was also found to exhibit an IC50 of around 74 nM using 1 mM cAMP as substrate. |
| Animal experiment [1]: | |
| Animal models | SCID mice |
| Dosage form | 5 mg·kg-1·day-1 ; oral taken |
| Application | The pharmacological activity of apremilast (5 mg·kg-1·day-1 total divided into 2 daily doses) was tested in comparison with cyclosporine (5 mg·kg-1·day-1 total divided into 2 daily doses) and vehicle control (0.1 mL·day-1 divided into twice daily doses) in a mouse xenograft model of psoriasis. Epidermal thickness and proliferation index correlated with histological findings and demonstrated significant differences between treatment groups. Notably, apremilast caused statistically significant reductions in epidermal thickness (P < 0.001) and proliferation index (P < 0.001). |
| Other notes | Please test the solubility of all compounds indoor, and the actual solubility may slightly differ with the theoretical value. This is caused by an experimental system error and it is normal. |
| References: [1] Schafer P H, Parton A, Gandhi A K, et al. Apremilast, a cAMP phosphodiesterase‐4 inhibitor, demonstrates anti‐inflammatory activity in vitro and in a model of psoriasis[J]. British journal of pharmacology, 2010, 159(4): 842-855. | |

Apremilast (CC-10004) Dilution Calculator

Apremilast (CC-10004) Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.1716 mL | 10.8578 mL | 21.7155 mL | 43.4311 mL | 54.2888 mL |
| 5 mM | 0.4343 mL | 2.1716 mL | 4.3431 mL | 8.6862 mL | 10.8578 mL |
| 10 mM | 0.2172 mL | 1.0858 mL | 2.1716 mL | 4.3431 mL | 5.4289 mL |
| 50 mM | 0.0434 mL | 0.2172 mL | 0.4343 mL | 0.8686 mL | 1.0858 mL |
| 100 mM | 0.0217 mL | 0.1086 mL | 0.2172 mL | 0.4343 mL | 0.5429 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Apremilast, also known as CC-10004, is a novel and potent small-molecule inhibitor of phosphodiesterase 4 (PDE4), a key enzyme involved in cyclic adenosine monophosphate (cAMP) degradation and cytokine production of inflammatory cells. Apremilast binds to the catalytic site of PDE4, with values of inhibition constant IC50 and affinity constant Kiof 0.074 μM and 68 nM respectively, consequently leading to the degradation of cAMP. Apremilast exhibits anti-inflammatory activities against inflammatory disease in animal models as well as human chronic inflammatory diseases (such as psoriasis and psoriatic arthritis) by blocking the synthesis of a range of pro-flammatory cytokines and chemokines, including tumor necrosis factor alpha, interleukin 23, CXCL9 and CXCL10.
Reference
Georg Schett, Victor S. Sloan, Randall M. Stevens and Peter Schafer. Apremilast: a novel PDE4 inhibitor in the treatment of autoimmune and inflammatory disease. Ther Adv Musculoskelet Dis. 2010; 2(5): 271-278
- 4-Methoxy-1-methoxycarbonyl-beta-carboline
Catalog No.:BCN1401
CAS No.:60807-25-2
- Lithium Citrate
Catalog No.:BCC3804
CAS No.:6080-58-6
- Sinomenine HCl
Catalog No.:BCN6318
CAS No.:6080-33-7
- Dulcitol
Catalog No.:BCN8153
CAS No.:608-66-2
- 5,7,8-Trimethoxycoumarin
Catalog No.:BCN4125
CAS No.:60796-65-8
- Norbraylin
Catalog No.:BCN4124
CAS No.:60796-64-7
- Berbamine hydrochloride
Catalog No.:BCN2400
CAS No.:6078-17-7
- Quercetin 3-O-beta-D-glucose-7-O-beta-D-gentiobioside
Catalog No.:BCN7821
CAS No.:60778-02-1
- SB742457
Catalog No.:BCC5058
CAS No.:607742-69-8
- A 77-01
Catalog No.:BCC1318
CAS No.:607737-87-1
- Ethyl 4-methoxysalicylate
Catalog No.:BCN3499
CAS No.:35031-00-6
- Meloside A
Catalog No.:BCN2278
CAS No.:60767-80-8
- Esculentoside B
Catalog No.:BCN5011
CAS No.:60820-94-2
- Crotaverrine
Catalog No.:BCN2142
CAS No.:60827-69-2
- Ligularidine
Catalog No.:BCN2141
CAS No.:60872-63-1
- PIR 3.5
Catalog No.:BCC6128
CAS No.:6088-51-3
- H-DL-Pro-OH
Catalog No.:BCC3026
CAS No.:609-36-9
- 6-Hydroxycoumarin
Catalog No.:BCC9207
CAS No.:6093-68-1
- YZ9
Catalog No.:BCC8001
CAS No.:6093-71-6
- Bz-Glu-OH
Catalog No.:BCC2922
CAS No.:6094-36-6
- Geraniin
Catalog No.:BCN2402
CAS No.:60976-49-0
- 2-Methoxycinnamic acid
Catalog No.:BCN5038
CAS No.:6099-03-2
- Dibucaine (Cinchocaine) HCl
Catalog No.:BCC3760
CAS No.:61-12-1
- Adenosine 5'-monophosphate
Catalog No.:BCC8809
CAS No.:61-19-8
Pharmacogenetics and Pharmacogenomics in Moderate-to-Severe Psoriasis.[Pubmed:28921458]
Am J Clin Dermatol. 2018 Apr;19(2):209-222.
Pharmacogenetics is the study of variations in DNA sequence related to drug response. Moreover, the evolution of biotechnology and the sequencing of human DNA have allowed the creation of pharmacogenomics, a branch of genetics that analyzes human genes, the RNAs and proteins encoded by them, and the inter-and intra-individual variations in expression and function in relation to drug response. Pharmacogenetics and pharmacogenomics are being used to search for biomarkers that can predict response to systemic treatments, including those for moderate-to-severe psoriasis. Psoriasis is a chronic inflammatory disease with an autoimmune contribution. Although its etiology remains unknown, genetic, epigenetic, and environmental factors play a role in its development. Diverse systemic and biologic therapies are used to treat moderate-to-severe psoriasis. However, these treatments are not curative, and patients exhibit a wide range of responses to them. Moderate-to-severe psoriasis is usually treated with systemic immunomodulators such as acitretin, ciclosporin, and methotrexate. Anti-tumor necrosis factor (TNF) drugs (adalimumab, etanercept, or infliximab) are the first-line treatment for patients resistant to conventional systemic therapies. Although these therapies are very efficient, around 30-50% of patients have inadequate response. Ustekinumab is a monoclonal antibody that targets interleukin (IL)-12 and IL-23 and is used for moderate-to-severe psoriasis. New drugs (apremilast, brodalumab, guselkumab, ixekizumab, and secukinumab) have recently been approved for psoriasis. However, response rates to systemic treatments for moderate-to-severe psoriasis range from 35 to 80%, so it is necessary to identify non-invasive biomarkers that could help predict treatment outcomes of these therapies and individualize care for patients with psoriasis. These biomarkers could improve patient quality of life and reduce health costs and potential side effects. Pharmacogenetic studies have identified potential biomarkers for response to biologic treatments for moderate-to-severe psoriasis. These biomarkers need to be validated in clinical trials involving large cohorts of patients before they can be translated to the clinic. We review pharmacogenetics and pharmacogenomics studies for the treatment of moderate-to-severe plaque psoriasis.
Apremilast in psoriasis - a prospective real-world study.[Pubmed:28925560]
J Eur Acad Dermatol Venereol. 2018 Feb;32(2):254-259.
BACKGROUND: Apremilast is a novel oral phosphodiesterase-4 inhibitor approved for psoriasis treatment. Randomized trials have documented its efficacy and safety, but data on real-world patients are scarce. OBJECTIVES: We aim to characterize psoriasis patients treated with apremilast in a real-world setting and calculate drug survival as an important measure of efficacy and compliance. METHODS: All patients with psoriasis who received apremilast between 1 April 2015 and 19 January 2017 were evaluated every 4 weeks, and we documented: age, weight, height, smoking status, family history of psoriasis, joint involvement, previous treatments, psoriasis area severity index (PASI) scores, and the onset and duration of adverse events (AE). Efficacy was analysed by PASI50, PASI75 and PASI90, reflecting the improvement of skin lesions compared to the PASI-baseline. Kaplan-Meier statistics were used for drug survival estimates. RESULTS: Forty-eight patients were included. The median apremilast drug survival was 12.5 weeks (range 1-87). Three patients (6.3%) reached PASI90, nine (18.8%) PASI75 and eight patients (16.7%) PASI50. Patient weight inversely correlated with a PASI50 response (P < 0.05, n = 37), and none of the obese patients (BMI > 30.0, n = 6) reached PASI75, compared to 32% of the non-obese patients (BMI < 30.0, n = 31). Thirty-one patients (64.6%) reported at least one AE, most frequently diarrhoea (n = 21, 43.8%), headache (n = 7, 14.6%) and joint pain (n = 5, 10.4%). CONCLUSIONS: Despite differences between real-world and trial patients, apremilast is safe and effective for the treatment of skin psoriasis in the daily practice. Up to 40% of patients will reach PASI50 or higher, but only few patients will reach PASI90. Bodyweight might affect drug efficacy.


