E4CPGGroup II/group I mGlu antagonist CAS# 170846-89-6 |
- L 006235
Catalog No.:BCC2361
CAS No.:294623-49-7
- E-64
Catalog No.:BCC1222
CAS No.:66701-25-5
- E-64-c
Catalog No.:BCC3588
CAS No.:76684-89-4
- Cysteine Protease inhibitor
Catalog No.:BCC5301
CAS No.:921625-62-9
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 170846-89-6 | SDF | Download SDF |
| PubChem ID | 3937355 | Appearance | Powder |
| Formula | C11H13NO4 | M.Wt | 223.23 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | H2O : < 0.1 mg/mL (insoluble) DMSO : < 1 mg/mL (insoluble or slightly soluble) | ||
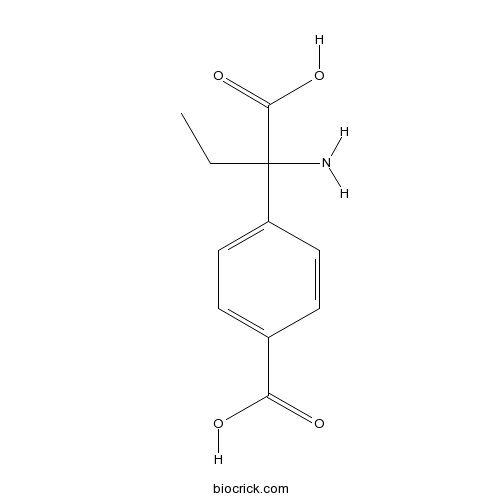
| Chemical Name | 4-(1-amino-1-carboxypropyl)benzoic acid | ||
| SMILES | CCC(C1=CC=C(C=C1)C(=O)O)(C(=O)O)N | ||
| Standard InChIKey | AIEFWRHRHFRLFT-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C11H13NO4/c1-2-11(12,10(15)16)8-5-3-7(4-6-8)9(13)14/h3-6H,2,12H2,1H3,(H,13,14)(H,15,16) | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Novel group I/group II metabotropic glutamate receptor antagonist, more potent than (RS)-MCPG. |

E4CPG Dilution Calculator

E4CPG Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 4.4797 mL | 22.3984 mL | 44.7968 mL | 89.5937 mL | 111.9921 mL |
| 5 mM | 0.8959 mL | 4.4797 mL | 8.9594 mL | 17.9187 mL | 22.3984 mL |
| 10 mM | 0.448 mL | 2.2398 mL | 4.4797 mL | 8.9594 mL | 11.1992 mL |
| 50 mM | 0.0896 mL | 0.448 mL | 0.8959 mL | 1.7919 mL | 2.2398 mL |
| 100 mM | 0.0448 mL | 0.224 mL | 0.448 mL | 0.8959 mL | 1.1199 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
E4CPG is a novel group I/group II metabotropic glutamate receptor antagonist, more potent than (RS)-MCPG .
References:
[1]. Sekiyama N et al. Structure-activity relationships of new agonists and antagonists of different metabotropic glutamate receptorsubtypes. Br J Pharmacol, 1996 Apr, 117(7):1493-503.
[2]. Bedingfield JS et al. Structure-activity relationships for a series of phenylglycine derivatives acting at metabotropic glutamate receptors (mGluRs). Br J Pharmacol. 1995 Dec;116(8):3323-9.
- CHPG
Catalog No.:BCC6910
CAS No.:170846-74-9
- Astressin
Catalog No.:BCC5790
CAS No.:170809-51-5
- Trityl candesartan cilexetil
Catalog No.:BCC9188
CAS No.:170791-09-0
- Aprepitant
Catalog No.:BCC1101
CAS No.:170729-80-3
- 11-Deoxymogroside V
Catalog No.:BCN8143
CAS No.:1707161-17-8
- Nociceptin
Catalog No.:BCC5686
CAS No.:170713-75-4
- 6beta-Hydroxyhispanone
Catalog No.:BCN7453
CAS No.:170711-93-0
- Bindone
Catalog No.:BCC8877
CAS No.:1707-95-5
- D-Mannitol diacetonide
Catalog No.:BCC8951
CAS No.:1707-77-3
- α-Conotoxin EI
Catalog No.:BCC5979
CAS No.:170663-33-9
- Fmoc-D-Abu-OH
Catalog No.:BCC3203
CAS No.:170642-27-0
- YC 1
Catalog No.:BCC7912
CAS No.:170632-47-0
- (RS)-APICA
Catalog No.:BCC6925
CAS No.:170847-18-4
- PNU 96415E
Catalog No.:BCC7467
CAS No.:170856-41-4
- Sonepiprazole
Catalog No.:BCC7879
CAS No.:170858-33-0
- Cyasterone
Catalog No.:BCN5416
CAS No.:17086-76-9
- Persianone
Catalog No.:BCN7359
CAS No.:170894-20-9
- Aburatubolactam A
Catalog No.:BCN1821
CAS No.:170894-24-3
- Donitriptan hydrochloride
Catalog No.:BCC7742
CAS No.:170911-68-9
- Dihydroactinidiolide
Catalog No.:BCN6890
CAS No.:17092-92-1
- Tetrindole mesylate
Catalog No.:BCC6763
CAS No.:170964-68-8
- EGLU
Catalog No.:BCC6871
CAS No.:170984-72-2
- H-Ser(tBu)-OMe.HCl
Catalog No.:BCC3033
CAS No.:17114-97-5
- PD 158780
Catalog No.:BCC7434
CAS No.:171179-06-9
BDNF-endocannabinoid interactions at neocortical inhibitory synapses require phospholipase C signaling.[Pubmed:24335212]
J Neurophysiol. 2014 Mar;111(5):1008-15.
Endogenous cannabinoids (endocannabinoids) and neurotrophins, particularly brain-derived neurotrophic factor (BDNF), are potent synaptic modulators that are expressed throughout the forebrain and play critical roles in many behavioral processes. Although the effects of BDNF at excitatory synapses have been well characterized, the mechanisms of action of BDNF at inhibitory synapses are not well understood. Previously we have found that BDNF suppresses presynaptic GABA release in layer 2/3 of the neocortex via postsynaptic tropomyosin-related kinase receptor B (trkB) receptor-induced release of endocannabinoids. To examine the intracellular signaling pathways that underlie this effect, we used pharmacological approaches and whole cell patch-clamp techniques in layer 2/3 pyramidal neurons of somatosensory cortex in brain slices from juvenile Swiss CD1 mice. Our results indicated that phospholipase Cgamma (PLCgamma) is involved in the CB1 receptor-mediated synaptic effect of BDNF, because the BDNF effect was blocked in the presence of the broad-spectrum PLC inhibitors U-73122 and edelfosine, whereas the inactive analog U-73343 did not alter the suppressive effect of BDNF at inhibitory synapses. Endocannabinoid release can also be triggered by metabotropic glutamate receptor (mGluR)-mediated activation of PLCbeta, and BDNF has been shown to enhance spontaneous glutamate release. An mGluR antagonist, E4CPG, however, did not block the BDNF effect. In addition, the effect of BDNF was independent of other signaling pathways downstream of trkB receptor activation, namely, mitogen-activated protein kinase and phosphoinositide 3-kinase pathways, as well as protein kinase C signaling.
Calcium-activated sustained firing responses distinguish accessory from main olfactory bulb mitral cells.[Pubmed:22553031]
J Neurosci. 2012 May 2;32(18):6251-62.
Many mammals rely on pheromones for mediating social interactions. Recent studies indicate that both the main olfactory system (MOS) and accessory olfactory system (AOS) detect and process pheromonal stimuli, yet the functional difference between these two chemosensory systems remains unclear. We hypothesized that the main functional distinction between the MOS and AOS is the type of sensory information processing performed by each system. Here we compared the electrophysiological responses of mitral cells recorded from the accessory olfactory bulb (AOB) and main olfactory bulb (MOB) in acute mouse brain slices to various stimuli and found them markedly different. The response of MOB mitral cells to brief (0.1 ms, 1-100 V) stimulation of their sensory afferents remained transient regardless of stimulus strength, whereas sufficiently strong stimuli evoked sustained firing in AOB mitral cells lasting up to several minutes. Using EPSC-like current injections (10-100 pA, 10 ms rise time constant, 5 s decay time constant) in the presence of various synaptic blockers (picrotoxin, CGP55845, APV, DNQX, E4CPG, and MSPG), we demonstrated that this difference is attributable to distinct intrinsic properties of the two neuronal populations. The AOB sustained responses were found to be mediated by calcium-activated nonselective cationic current induced by transient intense firing. This current was found to be at least partially mediated by TRPM4 channels activated by calcium influx. We hypothesize that the sustained activity of the AOS induces a new sensory state in the animal, reflecting its social context.
Ionotropic and metabotropic glutamate receptor mediation of glucocorticoid-induced apoptosis in hippocampal cells and the neuroprotective role of synaptic N-methyl-D-aspartate receptors.[Pubmed:12946705]
Neuroscience. 2003;121(1):123-31.
Glutamate receptors have been proposed to mediate the apoptotic actions of glucocorticoids in hippocampal cells. To further analyze the role of glutamate receptors in this process, we pretreated primary hippocampal cells from neonatal (postnatal day 4) rats with antagonists of ionotropic glutamate receptor (iGluR) and metabotropic glutamate receptor (mGluR) antagonists before exposure to the specific glucocorticoid receptor agonist dexamethasone (DEX) at a dose of 1 microM. Dizocilpine (MK801; a general N-methyl-D-aspartic acid [NMDA] receptor antagonist, NMDAR antagonist) and ifenprodil (a specific ligand of the NMDAR 2B subunit, NR2B), were used to block iGluR; (RS)-alpha-ethyl-4-carboxyphenylglycine (E4CPG) and (RS)-alpha-cyclopropyl-4-phosphonophenyl-glycine (CPPG) were employed as I/II (E4CPG) and II/III (CPPG) mGluR antagonists. Blockade of iGluR resulted in a significant attenuation of DEX-induced cell death; the finding that ifenprodil exerted a similar potency to MK801 demonstrates the involvement of NR2B receptors in glucocorticoid-induced cell death. Apoptosis accounted for a significant amount of the cell loss observed, as detected by terminal deoxynucleotidyl transferase-mediated dUTP nick-end labeling histochemistry for the in situ labeling of DNA breaks; apoptotic cells were distinguished from necrosis on the basis of morphological criteria, including chromatin condensation, membrane blebbing and presence of apoptotic bodies. Treatment with E4CPG and CPPG completely abolished the apoptotic response to DEX, thus showing the additional contribution of mGluR to the phenomenon. Further, dose-response studies with NMDA revealed that whereas high (10 microM) doses of NMDA themselves elicit cytotoxic responses, low (1-5 microM) concentrations of NMDA can effectively oppose DEX-induced cell death. Interestingly, the neuroprotective actions of low dose NMDA stimulation were abolished when either synaptic or extrasynaptic NMDA receptors were blocked with MK801 in combination with the GABA receptor antagonist bicuculline (synaptic) or ifenprodil (extrasynaptic). In summary, the present data show that both iGluR and mGluR mediate the neurotoxic effects of glucocorticoids on hippocampal cells and that pre-treatment with low doses of NMDA, by acting on synaptic and extrasynaptic receptors, render hippocampal cells less vulnerable to glucocorticoid insults.
Mechanisms underlying the nociception and paw oedema caused by injection of glutamate into the mouse paw.[Pubmed:11750907]
Brain Res. 2002 Jan 11;924(2):219-28.
This study characterizes the receptor subtypes and investigates some of the mechanisms by which glutamate, injected intraplantarly (i.pl.) into the mouse paw, produces nociception and paw oedema. I.pl. injection of glutamate induced a rapid-onset, dose-related pain response associated with oedema formation, with mean ED(50) values of 2.6 (1.6-4.3) and 0.5 (0.4-0.7) micromol/kg, respectively. Pretreatment with Chicago sky blue 6B (100 microg/kg), an inhibitor of glutamate uptake, caused a significant (about sixfold) reduction of the mean ED(50) value for glutamate-induced nociception, but not paw oedema. NMDA receptor antagonist MK 801, given by systemic (i.p.), intracerebroventricular (i.c.v.), i.pl. or intrathecal (i.t.) routes, produced graded inhibition of glutamate-induced nociception. Non-NMDA receptor antagonists NBQX or GAMS, metabotropic antagonist E4CPG, and also the antagonist that acts at the NMDA receptor-associated glycine binding site felbamate, significantly inhibited the nociception induced by glutamate. L(omega)-N-nitro-arginine (given i.p., i.t., i.pl. or i.c.v.) prevented the nociception and paw oedema caused by glutamate, an effect that was reversed by L-arginine but not by D-arginine. S-nitroso-N-acetyl-D,L-penicillamine (SNAP), given i.pl., greatly potentiated glutamate-induced nociception and oedema formation. Finally, the i.pl. injection of glutamate was accompanied by a graded increase in the nitrite levels of the hindpaw exudate. It is concluded that the nociception caused by i.pl. injection of glutamate probably involves the activation of NMDA and non-NMDA receptors by a mechanism which largely depends on the activation of L-arginine-nitric oxide pathway. Glutamate-induced paw oedema seems to be primarily mediated by non-NMDA ionotropic glutamate receptors and release of nitric oxide.
Structure-activity relationships for a series of phenylglycine derivatives acting at metabotropic glutamate receptors (mGluRs).[Pubmed:8719814]
Br J Pharmacol. 1995 Dec;116(8):3323-9.
1. The actions of a series of twelve phenylglycine derivatives at metabotropic glutamate receptors (mGluRs) linked to both phosphoinositide hydrolysis (PI) and cyclic AMP were investigated. 2. PI hydrolysis was determined by the accumulation of [3H]-inositol-monophosphate ([3H]-IP1) in neonatal ral cortical slices prelabelled with [3H]-myo-inositol. The non-selective mGluR agonist (1S,3R)-1-aminocyclopentane-1, 3-dicarboxylic acid ((1S,3R)-ACPD) produced a concentration-dependent increase in [3H]-IP1 (EC50 approximately 20 microM). This agonist was subsequently used to investigate potential antagonist activity of the phenylglycine derivatives. Of the compounds tested (+)-alpha-methyl-4-carboxyphenylglycine (M4CPG) and (RS)-alpha-ethyl-4-carboxyphenylglycine (E4CPG) were the most active with KP values of 0.184 +/- 0.04 mM and 0.367 +/- 0.2 mM respectively. 3. Activity at adenylyl cylase-coupled mGluRs was investigated by determining the accumulation of [3H]-cyclic AMP in adult rat cortical slices. [3H]-cyclic AMP accumulation, elicited by 30 microM forskolin, was inhibited by (2S,3S,4S)-alpha-(carboxycyclopropyl)glycine (L-CCG-1) and L-2-amino-4-phosphonobutanoate (L-AP4) with respective EC50 values of 0.3 microM and 10 microM. Neither agonist was able to inhibit completely forskolin stimulated cyclic AMP accumulation; this is evidence that not all adenylyl cyclase is susceptible to modulation by mGluRs. Phenylglycine derivatives were examined for their ability to antagonize the inhibition of [3H]-cyclic AMP accumulation by L-CCG-1 or L-AP4 at their EC50 concentrations. 4. A rank order of potency of the phenylglycine derivatives as antagonists of L-AP4 and L-CCG-1 was obtained. The most effective compound. (RS)-alpha-methyl-3-carboxymethylphenylglycine (M3CMPG) had IC50 values in the order of 1 microM against L-AP4 and 0.4 microM against L-CCG-1. 5. The results from this study indicate that phenylglycine-derived compounds can discriminate between groups of metabotropic glutamate receptors and may also display some selective activity between subtypes within groups. Future work based on these findings may lead to the development of more selective and potent compounds as important pharmacological tools.
Structure-activity relationships of new agonists and antagonists of different metabotropic glutamate receptor subtypes.[Pubmed:8730745]
Br J Pharmacol. 1996 Apr;117(7):1493-503.
1. We investigated the agonist and antagonist activities of 22 new phenylglycine and phenylalanine derivatives for metabotropic glutamate receptors (mGluRs) by examining their effects on the signal transduction of mGluR1, mGluR2 and mGluR6 subtypes expressed in Chinese hamster ovary cells. This analysis revealed several structural characteristics that govern receptor subtype specificity of the agonist and antagonist activities of phenylglycine derivatives. 2. Hydroxyphenylglycine derivatives possessed either an agonist activity on mGluR1/mGluR6 or an antagonist activity on mGluR1. 3. Carboxyphenylglycine derivatives showed an agonist activity on mGluR2 but an antagonist activity on mGluR1. 4. alpha-Methylation or alpha-ethylation of the carboxyphenylglycine derivatives converts the agonist property for mGluR2 to an antagonist property, thus producing antagonists at both mGluR1 and mGluR2. 5. Structurally-corresponding phenylalanine derivatives showed little or no agonist or antagonist activity on any subtypes of the receptors. 6. This investigation demonstrates that the nature and positions of side chains and ring substituents incorporated into the phenylglycine structure are critical in determining the agonist and antagonist activities of members of this group of compounds on different subtypes of the mGluR family. 7. We also tested two alpha-methyl derivatives of mGluR agonists. (2S, 1'S, 2'S)-2-(2-Carboxycyclopropyl)glycine (L-CCG-I) is a potent agonist for mGluR2 but alpha-methylation of this compound changes its activity to that of an mGluR2-selective antagonist. In contrast, alpha-methylation of L-2-amino-4-phosphonobutyrate (L-AP4) results in retention of an agonist activity on mGluR6. Thus, alpha-methylation produces different effects, depending on the chemical structures of lead compounds and/or on the subtype of mGluR tested.


