PNU 96415E5-HT2A and D4 antagonist; antipsychotic CAS# 170856-41-4 |
- PF-562271
Catalog No.:BCC3674
CAS No.:717907-75-0
- TAE226 (NVP-TAE226)
Catalog No.:BCC3885
CAS No.:761437-28-9
- PF-573228
Catalog No.:BCC4496
CAS No.:869288-64-2
- PF-00562271
Catalog No.:BCC3684
CAS No.:939791-38-5
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 170856-41-4 | SDF | Download SDF |
| PubChem ID | 9909647 | Appearance | Powder |
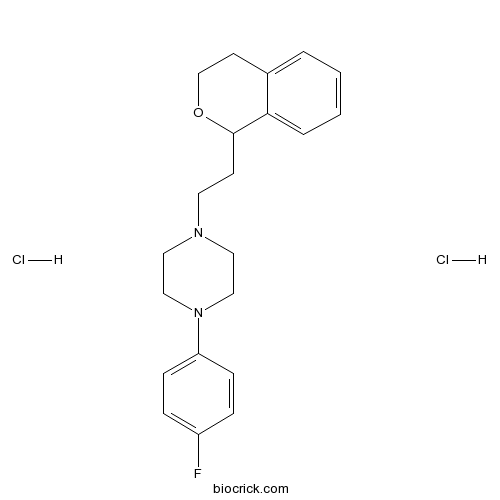
| Formula | C21H27Cl2FN2O | M.Wt | 413.36 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble to 10 mM in water | ||
| Chemical Name | 1-[2-(3,4-dihydro-1H-isochromen-1-yl)ethyl]-4-(4-fluorophenyl)piperazine;dihydrochloride | ||
| SMILES | C1COC(C2=CC=CC=C21)CCN3CCN(CC3)C4=CC=C(C=C4)F.Cl.Cl | ||
| Standard InChIKey | OGMGYKPECFQXJJ-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C21H25FN2O.2ClH/c22-18-5-7-19(8-6-18)24-14-12-23(13-15-24)11-9-21-20-4-2-1-3-17(20)10-16-25-21;;/h1-8,21H,9-16H2;2*1H | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Antipsychotic agent. Displays high affinity for dopamine D4 and serotonergic 5-HT2A receptors and relatively weak affinity at D2 receptors (Ki values are 3.0, 5.8, 134, 181, 199, 240, 411 and > 678 nM for D4, 5-HT2A, 5-HT1A, α1, D2, D3, D1, α2 and muscarinic receptors respectively). Inhibits exploratory locomotor activity and antagonizes d-amphetamine-induced locomotor stimulation in rats. |

PNU 96415E Dilution Calculator

PNU 96415E Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.4192 mL | 12.096 mL | 24.192 mL | 48.384 mL | 60.48 mL |
| 5 mM | 0.4838 mL | 2.4192 mL | 4.8384 mL | 9.6768 mL | 12.096 mL |
| 10 mM | 0.2419 mL | 1.2096 mL | 2.4192 mL | 4.8384 mL | 6.048 mL |
| 50 mM | 0.0484 mL | 0.2419 mL | 0.4838 mL | 0.9677 mL | 1.2096 mL |
| 100 mM | 0.0242 mL | 0.121 mL | 0.2419 mL | 0.4838 mL | 0.6048 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- (RS)-APICA
Catalog No.:BCC6925
CAS No.:170847-18-4
- E4CPG
Catalog No.:BCC6888
CAS No.:170846-89-6
- CHPG
Catalog No.:BCC6910
CAS No.:170846-74-9
- Astressin
Catalog No.:BCC5790
CAS No.:170809-51-5
- Trityl candesartan cilexetil
Catalog No.:BCC9188
CAS No.:170791-09-0
- Aprepitant
Catalog No.:BCC1101
CAS No.:170729-80-3
- 11-Deoxymogroside V
Catalog No.:BCN8143
CAS No.:1707161-17-8
- Nociceptin
Catalog No.:BCC5686
CAS No.:170713-75-4
- 6beta-Hydroxyhispanone
Catalog No.:BCN7453
CAS No.:170711-93-0
- Bindone
Catalog No.:BCC8877
CAS No.:1707-95-5
- D-Mannitol diacetonide
Catalog No.:BCC8951
CAS No.:1707-77-3
- α-Conotoxin EI
Catalog No.:BCC5979
CAS No.:170663-33-9
- Sonepiprazole
Catalog No.:BCC7879
CAS No.:170858-33-0
- Cyasterone
Catalog No.:BCN5416
CAS No.:17086-76-9
- Persianone
Catalog No.:BCN7359
CAS No.:170894-20-9
- Aburatubolactam A
Catalog No.:BCN1821
CAS No.:170894-24-3
- Donitriptan hydrochloride
Catalog No.:BCC7742
CAS No.:170911-68-9
- Dihydroactinidiolide
Catalog No.:BCN6890
CAS No.:17092-92-1
- Tetrindole mesylate
Catalog No.:BCC6763
CAS No.:170964-68-8
- EGLU
Catalog No.:BCC6871
CAS No.:170984-72-2
- H-Ser(tBu)-OMe.HCl
Catalog No.:BCC3033
CAS No.:17114-97-5
- PD 158780
Catalog No.:BCC7434
CAS No.:171179-06-9
- N-Methylquipazine dimaleate
Catalog No.:BCC6697
CAS No.:171205-17-7
- Posaconazole
Catalog No.:BCC1103
CAS No.:171228-49-2
PNU-96415E, a potential antipsychotic agent with clozapine-like pharmacological properties.[Pubmed:9103528]
J Pharmacol Exp Ther. 1997 Apr;281(1):440-7.
The atypical antipsychotic drug clozapine interacts with multiple transmitter systems, among them the D4 subtype of dopamine receptors. PNU-96415E is chemically unrelated to clozapine and has its highest binding affinity for the D4 and 5-HT2A receptors. In comparison to clozapine, PNU-96415E is weaker in binding to D1, D2, alpha1 and muscarinic receptors. PNU-96415E inhibited exploratory locomotor activity in mice and rats, and antagonized d-amphetamine-induced locomotor stimulation in rats. It antagonized apomorphine-induced cage climbing, and blocked head and body twitch produced by 5-HTP in mice. Like clozapine, but unlike haloperidol, PNU-96415E did not antagonize stereotypic behaviors produced by a high dose of d-amphetamine or methylphenidate in rats and mice. PNU-96415E blocked conditioned avoidance in rats but produced no catalepsy, a pattern similar to clozapine but different from haloperidol. In rats trained to discriminate clozapine from saline injections, the stimulus effect generalized completely with PNU-96415E, but not haloperidol. This profile of pharmacological activities is consistent with that of an atypical antipsychotic and, as in the case with clozapine, the behavioral effects of PNU-96415E cannot be ascribed to a single receptor mechanism.
Discriminative stimulus properties of the atypical neuroleptic clozapine in rats: tests with subtype selective receptor ligands.[Pubmed:9890260]
Behav Pharmacol. 1998 Dec;9(8):699-710.
The interoceptive stimulus induced by clozapine (5 mg/kg, i.p.) has been characterized in an operant drug discrimination procedure in the rat using a wide range of receptor subtype-selective agonists and antagonists. Only the muscarinic receptor antagonist scopolamine generalized fully to clozapine (>80%). Partial generalization (defined here as 40% maximal generalization) was seen with the D1 receptor antagonist SCH 23390 (43% maximal generalization), the alpha1-adrenoceptor antagonist prazosin (67%) and the alpha2-adrenoceptor antagonist methoxyidazoxan (42%). All other specific agents tested induced <25% maximal generalization, including the alpha2-adrenoceptor antagonist yohimbine (24%), the histamine H1 receptor antagonist mepyramine (21%), the D2 antagonist typical neuroleptic haloperidol (23%), the D4 receptor antagonist L-745,870 (14%), the 5-hydroxytryptamine-1A (5-HT1A) receptor agonist S-14506 (8%), the 5-HT2A receptor antagonists ketanserin (0%) and M100907 (12%), the 5-HT2B/2C receptor antagonists SB 200646A (8%) and SDZ SER 082 (6%), and the 5-HT3 receptor antagonist ondansetron (0%). The clozapine discriminative stimulus was not blocked by the dopamine D1 receptor antagonist SCH 23390, or by the 5-HT1A receptor antagonist WAY 100635, when given concomitantly with clozapine. Although the results suggest that muscarinic antagonism plays a major role in the clozapine cue, the results have to be considered in the light of the full generalization to clozapine seen with various antipsychotic agents which have very low affinity for muscarinic receptors, including zotepine, quetiapine, JL13 and PNU 96415 (a finding replicated in rats from the same breeding colony as those which generalized to scopolamine). Thus, generalization to clozapine for antipsychotics with multiple affinities but with low muscarinic affinity is probably mediated by additive or perhaps supra-additive actions at other receptors, although extensive studies with various combinations of drug mixtures are required to validate this hypothesis.


