ErgosterolCAS# 57-87-4 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 57-87-4 | SDF | Download SDF |
| PubChem ID | 444679 | Appearance | Powder |
| Formula | C28H44O | M.Wt | 396.7 |
| Type of Compound | Steroids | Storage | Desiccate at -20°C |
| Solubility | Ethanol : 2.6 mg/mL (6.55 mM; ultrasonic and warming and heat to 80°C) | ||
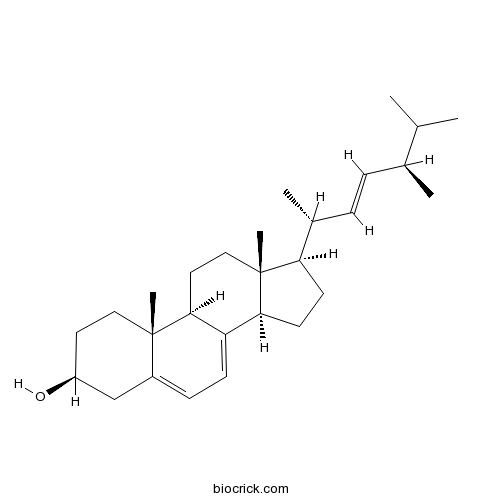
| Chemical Name | (3S,9S,10R,13R,14R,17R)-17-[(E,2R,5R)-5,6-dimethylhept-3-en-2-yl]-10,13-dimethyl-2,3,4,9,11,12,14,15,16,17-decahydro-1H-cyclopenta[a]phenanthren-3-ol | ||
| SMILES | CC(C)C(C)C=CC(C)C1CCC2C1(CCC3C2=CC=C4C3(CCC(C4)O)C)C | ||
| Standard InChIKey | DNVPQKQSNYMLRS-APGDWVJJSA-N | ||
| Standard InChI | InChI=1S/C28H44O/c1-18(2)19(3)7-8-20(4)24-11-12-25-23-10-9-21-17-22(29)13-15-27(21,5)26(23)14-16-28(24,25)6/h7-10,18-20,22,24-26,29H,11-17H2,1-6H3/b8-7+/t19-,20+,22-,24+,25-,26-,27-,28+/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Ergosterol is the primary sterol found in fungi, with antioxidative, anti-proliferative, and anti-inflammatory effects, ergosterol peroxide has the potential to be developed as a therapeutic agent to prevent renal fibrosis. Ergosterol can promote pheromone signaling and plasma membrane fusion in mating yeast, the critical requirement for ergosterol in V-ATPase function may underlie the antifungal activity of azoles. |
| Targets | TGF-β/Smad | ERK | p38MAPK | JNK | ATPase | Calcium Channel | Antifection |
| In vitro | Ergosterol peroxide from Cordyceps cicadae ameliorates TGF-β1-induced activation of kidney fibroblasts.[Pubmed: 24095053]Phytomedicine. 2014 Feb 15;21(3):372-8.Chronic kidney disease is a growing public health problem with an urgent need for new pharmacological agents. Ergosterol peroxide (EP) is the major sterol produced by Cordyceps cicadae Shing (C. cicadae), a widely used traditional Chinese medicine. C. cicadae has been used to treat many kinds of diseases and has a potential benefit on renoprotection.
This study aimed to investigate the anti-fibrotic effects of EP as well as the underlying mechanisms.
Trans-chalcone and quercetin down-regulate fatty acid synthase gene expression and reduce ergosterol content in the human pathogenic dermatophyte Trichophyton rubrum.[Pubmed: 24044691]BMC Complement Altern Med. 2013 Sep 17;13:229.Fatty acid synthase (FAS) is a promising antifungal target due to its marked structural differences between fungal and mammalian cells. The aim of this study was to evaluate the antifungal activity of flavonoids described in the scientific literature as FAS inhibitors (quercetin, trans-chalcone, ellagic acid, luteolin, galangin, and genistein) against the dermatophyte Trichophyton rubrum and their effects on fatty acid and Ergosterol synthesis.
Ergosterol as a measure of living fungal biomass: persistence in environmental samples after fungal death.[Pubmed: 15369861 ]J Microbiol Methods. 2004 Nov;59(2):253-62.The membrane lipid Ergosterol is found almost exclusively in fungi, and is frequently used by environmental microbiologists as an indicator of living fungal biomass, based on the assumption that Ergosterol is labile, and therefore rapidly degraded after the death of fungal hyphae.
|
| In vivo | Requirement for ergosterol in V-ATPase function underlies antifungal activity of azole drugs.[Pubmed: 20532216]PLoS Pathog. 2010 Jun 3;6(6):e1000939.Ergosterol is an important constituent of fungal membranes. Azoles inhibit Ergosterol biosynthesis, although the cellular basis for their antifungal activity is not understood. We used multiple approaches to demonstrate a critical requirement for Ergosterol in vacuolar H(+)-ATPase function, which is known to be essential for fungal virulence.
|
| Cell Research | Ergosterol promotes pheromone signaling and plasma membrane fusion in mating yeast.[Pubmed: 18299351]J Cell Biol. 2008 Feb 25;180(4):813-26.Ergosterol depletion independently inhibits two aspects of yeast mating: pheromone signaling and plasma membrane fusion. In signaling, Ergosterol participates in the recruitment of Ste5 to a polarized site on the plasma membrane. Ergosterol is thought to form microdomains within the membrane by interacting with the long acyl chains of sphingolipids.
|

Ergosterol Dilution Calculator

Ergosterol Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.5208 mL | 12.604 mL | 25.208 mL | 50.4159 mL | 63.0199 mL |
| 5 mM | 0.5042 mL | 2.5208 mL | 5.0416 mL | 10.0832 mL | 12.604 mL |
| 10 mM | 0.2521 mL | 1.2604 mL | 2.5208 mL | 5.0416 mL | 6.302 mL |
| 50 mM | 0.0504 mL | 0.2521 mL | 0.5042 mL | 1.0083 mL | 1.2604 mL |
| 100 mM | 0.0252 mL | 0.126 mL | 0.2521 mL | 0.5042 mL | 0.6302 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Testosterone propionate
Catalog No.:BCC9172
CAS No.:57-85-2
- Progesterone
Catalog No.:BCN2198
CAS No.:57-83-0
- Sulfamethazine
Catalog No.:BCC4942
CAS No.:57-68-1
- Sulfaguanidine
Catalog No.:BCC4727
CAS No.:57-67-0
- Probenecid
Catalog No.:BCC4832
CAS No.:57-66-9
- Ethinyl Estradiol
Catalog No.:BCC3777
CAS No.:57-63-6
- Chlorotetracycline
Catalog No.:BCC8913
CAS No.:57-62-5
- Sucrose
Catalog No.:BCN5780
CAS No.:57-50-1
- Fructose
Catalog No.:BCN4969
CAS No.:57-48-7
- Esromiotin
Catalog No.:BCC8325
CAS No.:57-47-6
- Phenytoin
Catalog No.:BCC5070
CAS No.:57-41-0
- Benactyzine hydrochloride
Catalog No.:BCC8841
CAS No.:57-37-4
- Cholesterol
Catalog No.:BCN2199
CAS No.:57-88-5
- α-Estradiol
Catalog No.:BCC7497
CAS No.:57-91-0
- (+)-Tubocurarine chloride
Catalog No.:BCC7496
CAS No.:57-94-3
- Europine
Catalog No.:BCN1976
CAS No.:570-19-4
- Stigmasta-5,8-dien-3-ol
Catalog No.:BCN5769
CAS No.:570-72-9
- Tricine
Catalog No.:BCN5337
CAS No.:5704-04-1
- Paprotrain
Catalog No.:BCC7978
CAS No.:57046-73-8
- Isosativenediol
Catalog No.:BCN7458
CAS No.:57079-92-2
- 4'-Hydroxywogonin
Catalog No.:BCN5770
CAS No.:57096-02-3
- Boc-D-Lys(2-Cl-Z)-OH
Catalog No.:BCC3421
CAS No.:57096-11-4
- 5α-Androstane-3β,17β-diol
Catalog No.:BCC8751
CAS No.:571-20-0
- 8-Methoxykaempferol
Catalog No.:BCN3344
CAS No.:571-74-4
Ergosterol as a measure of living fungal biomass: persistence in environmental samples after fungal death.[Pubmed:15369861]
J Microbiol Methods. 2004 Nov;59(2):253-62.
The membrane lipid Ergosterol is found almost exclusively in fungi, and is frequently used by environmental microbiologists as an indicator of living fungal biomass, based on the assumption that Ergosterol is labile, and therefore rapidly degraded after the death of fungal hyphae. We studied the degradation of Ergosterol in environmental samples without living fungi. Under the conditions used in this study, Ergosterol was very stable both when added as a pure compound and when associated with dead fungi. The decrease of Ergosterol was at most 34% during 2 months when protected from sunlight. Presence of a natural bacterial assemblage did not enhance degradation over this time period, as compared to sterile controls. However, photochemical degradation was significant, and led to a 43% decrease of in Ergosterol content during 24 h. These results suggest that Ergosterol should be used cautiously as a biomarker for living fungi.
Ergosterol peroxide from Cordyceps cicadae ameliorates TGF-beta1-induced activation of kidney fibroblasts.[Pubmed:24095053]
Phytomedicine. 2014 Feb 15;21(3):372-8.
Chronic kidney disease is a growing public health problem with an urgent need for new pharmacological agents. Ergosterol peroxide (EP) is the major sterol produced by Cordyceps cicadae Shing (C. cicadae), a widely used traditional Chinese medicine. C. cicadae has been used to treat many kinds of diseases and has a potential benefit on renoprotection. This study aimed to investigate the anti-fibrotic effects of EP as well as the underlying mechanisms. A normal rat kidney fibroblast cell line (NRK-49F) was stimulated to undergo fibroblast activation by transforming growth factor-beta1 (TGF-beta1) and EP treatment was applied to explore its potential anti-fibrotic effects. Cell proliferation was investigated using MTT analysis. Fibrosis-associated protein expression was analyzed using immunohistochemistry and/or Western blotting. EP treatment attenuated TGF-beta1-induced renal fibroblast proliferation, expression of cytoskeleton protein and CTGF, as well as ECM production. Additionally, EP blocked TGF-beta1-stimulated phosphorylation of ERK1/2, p38 and JNK pathway. Moreover, the TGF-beta1-induced expression of fibronectin was attenuated by either inhibition of MAPKs or by EP treatment. In conclusion, our findings demonstrate that EP is able to suppress TGF-beta1-induced fibroblasts activation in NRK-49F. This new information provides a line of theoretical evidence supporting the use of C. cicadae in the intervention of kidney disease and suggests that EP has the potential to be developed as a therapeutic agent to prevent renal fibrosis.
Ergosterol promotes pheromone signaling and plasma membrane fusion in mating yeast.[Pubmed:18299351]
J Cell Biol. 2008 Feb 25;180(4):813-26.
Ergosterol depletion independently inhibits two aspects of yeast mating: pheromone signaling and plasma membrane fusion. In signaling, Ergosterol participates in the recruitment of Ste5 to a polarized site on the plasma membrane. Ergosterol is thought to form microdomains within the membrane by interacting with the long acyl chains of sphingolipids. We find that although sphingolipid-free Ergosterol is concentrated at sites of cell-cell contact, transmission of the pheromone signal at contact sites depends on a balanced ratio of Ergosterol to sphingolipids. If a mating pair forms between Ergosterol-depleted cells despite the attenuated pheromone response, the subsequent process of membrane fusion is retarded. Prm1 also participates in membrane fusion. However, Ergosterol and Prm1 have independent functions and only prm1 mutant mating pairs are susceptible to contact-dependent lysis. In contrast to signaling, plasma membrane fusion is relatively insensitive to sphingolipid depletion. Thus, the sphingolipid-free pool of Ergosterol promotes plasma membrane fusion.
Trans-chalcone and quercetin down-regulate fatty acid synthase gene expression and reduce ergosterol content in the human pathogenic dermatophyte Trichophyton rubrum.[Pubmed:24044691]
BMC Complement Altern Med. 2013 Sep 17;13:229.
BACKGROUND: Fatty acid synthase (FAS) is a promising antifungal target due to its marked structural differences between fungal and mammalian cells. The aim of this study was to evaluate the antifungal activity of flavonoids described in the scientific literature as FAS inhibitors (quercetin, trans-chalcone, ellagic acid, luteolin, galangin, and genistein) against the dermatophyte Trichophyton rubrum and their effects on fatty acid and Ergosterol synthesis. METHODS: The antifungal activity of the natural products was tested by the microdilution assay for determination of the minimum inhibitory concentration (MIC). The effect of the compounds on the cell membrane was evaluated using a protoplast regeneration assay. Ergosterol content was quantified by spectrophotometry. Inhibition of FAS by flavonoids was evaluated by an enzymatic assay to determine IC50 values. Quantitative RT-PCR was used to measure transcription levels of the FAS1 and ERG6 genes involved in fatty acid and Ergosterol biosynthesis, respectively, during exposure of T. rubrum to the flavonoids tested. RESULTS: The flavonoids quercetin and trans-chalcone were effective against T. rubrum, with MICs of 125 and 7.5 mug/mL for the wild-type strain (MYA3108) and of 63 and 1.9 mug/mL for the ABC transporter mutant strain (DeltaTruMDR2), respectively. The MICs of the fluconazole and cerulenin controls were 63 and 125 mug/mL for the wild-type strain and 30 and 15 mug/mL for the mutant strain, respectively. Quercetin and trans-chalcone also reduced Ergosterol content in the two strains, indicating that interference with fatty acid and Ergosterol synthesis caused cell membrane disruption. The MIC of quercetin reduced the number of regenerated protoplasts by 30.26% (wild-type strain) and by 91.66% (mutant strain). Half the MIC (0.5 MIC) of quercetin did not reduce the number of regenerated wild-type fungal colonies, but caused a 36.19% reduction in the number of mutant strain protoplasts. In contrast, the MIC and 0.5 MIC of trans-chalcone and cerulenin drastically reduced protoplast regeneration in the two strains. The FAS1 gene was repressed in the presence of MICs of quercetin, trans-chalcone, fluconazole and cerulenin. The ERG6 gene was induced in the presence of MICs of fluconazole and cerulenin and was repressed in the presence of MICs of trans-chalcone and quercetin. Trans-chalcone and quercetin inhibited the enzymatic activity of FAS, with IC50 values of 68.23 and 17.1 mug/mL, respectively. CONCLUSION: Trans-chalcone and quercetin showed antifungal activity against T. rubrum, reducing Ergosterol levels and modulating the expression of FAS1 and ERG6.
Requirement for ergosterol in V-ATPase function underlies antifungal activity of azole drugs.[Pubmed:20532216]
PLoS Pathog. 2010 Jun 3;6(6):e1000939.
Ergosterol is an important constituent of fungal membranes. Azoles inhibit Ergosterol biosynthesis, although the cellular basis for their antifungal activity is not understood. We used multiple approaches to demonstrate a critical requirement for Ergosterol in vacuolar H(+)-ATPase function, which is known to be essential for fungal virulence. Ergosterol biosynthesis mutants of S. cerevisiae failed to acidify the vacuole and exhibited multiple vma(-) phenotypes. Extraction of Ergosterol from vacuolar membranes also inactivated V-ATPase without disrupting membrane association of its subdomains. In both S. cerevisiae and the fungal pathogen C. albicans, fluconazole impaired vacuolar acidification, whereas concomitant Ergosterol feeding restored V-ATPase function and cell growth. Furthermore, fluconazole exacerbated cytosolic Ca(2+) and H(+) surges triggered by the antimicrobial agent amiodarone, and impaired Ca(2+) sequestration in purified vacuolar vesicles. These findings provide a mechanistic basis for the synergy between azoles and amiodarone observed in vitro. Moreover, we show the clinical potential of this synergy in treatment of systemic fungal infections using a murine model of Candidiasis. In summary, we demonstrate a new regulatory component in fungal V-ATPase function, a novel role for Ergosterol in vacuolar ion homeostasis, a plausible cellular mechanism for azole toxicity in fungi, and preliminary in vivo evidence for synergism between two antifungal agents. New insights into the cellular basis of azole toxicity in fungi may broaden therapeutic regimens for patient populations afflicted with systemic fungal infections.


