PaprotrainReversible inhibitor of MKLP-2 CAS# 57046-73-8 |
- CUDC-101
Catalog No.:BCC2149
CAS No.:1012054-59-9
- Vorinostat (SAHA, MK0683)
Catalog No.:BCC2145
CAS No.:149647-78-9
- ITF2357 (Givinostat)
Catalog No.:BCC2150
CAS No.:732302-99-7
- PCI-24781 (CRA-024781)
Catalog No.:BCC2155
CAS No.:783355-60-2
- JNJ-26481585
Catalog No.:BCC2147
CAS No.:875320-29-9
- AR-42 (OSU-HDAC42)
Catalog No.:BCC2161
CAS No.:935881-37-1
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 57046-73-8 | SDF | Download SDF |
| PubChem ID | 67514345 | Appearance | Powder |
| Formula | C16H11N3 | M.Wt | 245.28 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | DMSO : ≥ 100 mg/mL (407.70 mM) *"≥" means soluble, but saturation unknown. | ||
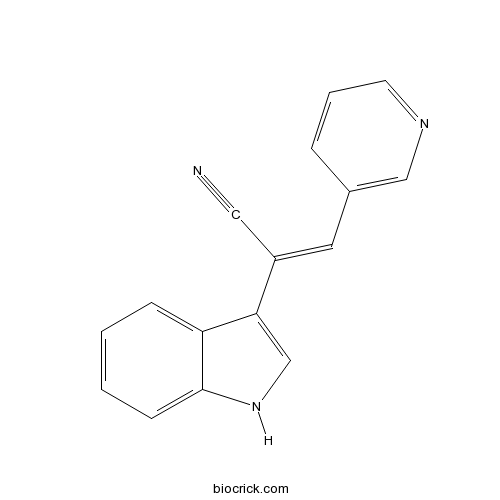
| Chemical Name | 2-(1H-indol-3-yl)-3-pyridin-3-ylprop-2-enenitrile | ||
| SMILES | C1=CC=C2C(=C1)C(=CN2)C(=CC3=CN=CC=C3)C#N | ||
| Standard InChIKey | YMYYQRZCCKBFBE-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C16H11N3/c17-9-13(8-12-4-3-7-18-10-12)15-11-19-16-6-2-1-5-14(15)16/h1-8,10-11,19H | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Reversible, non-ATP competitive inhibitor of mitotic kinesin-like protein 2 (MKLP-2) (Ki = 3.4 μM). Inhibits the basal ATPase activity of MKLP-2 (IC50 = 1.35 μM). Exhibits selectivity for MKLP-2 over 12 other members of the kinesin superfamily, including the closely-related MKLP-1. Cell permeable. |

Paprotrain Dilution Calculator

Paprotrain Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 4.077 mL | 20.3849 mL | 40.7697 mL | 81.5395 mL | 101.9243 mL |
| 5 mM | 0.8154 mL | 4.077 mL | 8.1539 mL | 16.3079 mL | 20.3849 mL |
| 10 mM | 0.4077 mL | 2.0385 mL | 4.077 mL | 8.1539 mL | 10.1924 mL |
| 50 mM | 0.0815 mL | 0.4077 mL | 0.8154 mL | 1.6308 mL | 2.0385 mL |
| 100 mM | 0.0408 mL | 0.2038 mL | 0.4077 mL | 0.8154 mL | 1.0192 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Paprotrain is a cell-permeable inhibitor of the kinesin MKLP-2, inhibits the ATPase activity of MKLP-2 with an IC50 of 1.35 μM and a Ki of 3.36 μM and shows a moderate inhibition activity on DYRK1A with an IC50 of 5.5 μM.
In Vitro:Paprotrain has been screened on a panel of CNS kinases. While inactive (IC50 >10 μM) on CDK5 and GSK3, it has shown a moderate activity on DYRK1A (IC50=5.5 μM)[1]. Time-lapse microscopy shows that disrupting MKlp2 expression with paprotrain results in polar body extrusion failure. This could be rescued after rescuing oocytes from paprotrain in fresh medium. Cell cycle analysis shows that most oocytes are arrested at metaphase I or telophase I. However, oocyte spindle structure and chromosome alignment are not disrupted after the inhibition of MKlp2 by paprotrain[2]. Paprotrain-treated porcine oocytes suffer failure of nuclear maturation. The number of oocytes arrested at early MI stage increase in a dose-dependent manner after KIF20A activity inhibition, while the percentage of oocytes that reach ATI and MII stages decrease after treatment[3].
References:
[1]. Labrière C, et al. Further investigation of Paprotrain: Towards the conception of selective and multi-targeted CNS kinase inhibitors. Eur J Med Chem. 2016 Nov 29;124:920-934.
[2]. Liu J, et al. MKlp2 inhibitor paprotrain affects polar body extrusion during mouse oocyte maturation. Reprod Biol Endocrinol. 2013 Dec 21;11:117.
[3]. Zhang Y, et al. KIF20A regulates porcine oocyte maturation and early embryo development.
[4]. Tcherniuk S, et al. Relocation of Aurora B and survivin from centromeres to the central spindle impaired by a kinesin-specific MKLP-2 inhibitor. Angew Chem Int Ed Engl. 2010 Oct 25;49(44):8228-31.
- Tricine
Catalog No.:BCN5337
CAS No.:5704-04-1
- Stigmasta-5,8-dien-3-ol
Catalog No.:BCN5769
CAS No.:570-72-9
- Europine
Catalog No.:BCN1976
CAS No.:570-19-4
- (+)-Tubocurarine chloride
Catalog No.:BCC7496
CAS No.:57-94-3
- α-Estradiol
Catalog No.:BCC7497
CAS No.:57-91-0
- Cholesterol
Catalog No.:BCN2199
CAS No.:57-88-5
- Ergosterol
Catalog No.:BCN5787
CAS No.:57-87-4
- Testosterone propionate
Catalog No.:BCC9172
CAS No.:57-85-2
- Progesterone
Catalog No.:BCN2198
CAS No.:57-83-0
- Sulfamethazine
Catalog No.:BCC4942
CAS No.:57-68-1
- Sulfaguanidine
Catalog No.:BCC4727
CAS No.:57-67-0
- Probenecid
Catalog No.:BCC4832
CAS No.:57-66-9
- Isosativenediol
Catalog No.:BCN7458
CAS No.:57079-92-2
- 4'-Hydroxywogonin
Catalog No.:BCN5770
CAS No.:57096-02-3
- Boc-D-Lys(2-Cl-Z)-OH
Catalog No.:BCC3421
CAS No.:57096-11-4
- 5α-Androstane-3β,17β-diol
Catalog No.:BCC8751
CAS No.:571-20-0
- 8-Methoxykaempferol
Catalog No.:BCN3344
CAS No.:571-74-4
- Laropiprant
Catalog No.:BCC1688
CAS No.:571170-77-9
- Erastin
Catalog No.:BCC4497
CAS No.:571203-78-6
- 16,17-Dihydroapovincamine
Catalog No.:BCN8049
CAS No.:57130-30-0
- Naftopidil
Catalog No.:BCC4352
CAS No.:57149-07-2
- Naftopidil DiHCl
Catalog No.:BCC4355
CAS No.:57149-08-3
- Paxilline
Catalog No.:BCC7235
CAS No.:57186-25-1
- Neosenkirkine
Catalog No.:BCN2138
CAS No.:57194-70-4
MKlp2 inhibitor paprotrain affects polar body extrusion during mouse oocyte maturation.[Pubmed:24359300]
Reprod Biol Endocrinol. 2013 Dec 21;11:117.
BACKGROUND: Mammalian oocyte meiotic maturation involves a number of important processes, including spindle assembly and migration, cortical reorganization and polar body extrusion. Numerous proteins contribute to these processes, but it is unknown whether MKlp2 (mitotic kinesin-like protein 2; also called KIF20A), a microtubule-associated protein that regulates cytokinesis during mitosis, is involved in oocyte maturation. METHODS: Confocal microscopy, time lapse microscopy, inhibitor treatment were adopted to examine the roles of MKlp2 in mouse oocyte. RESULTS: Immunostaining results showed that MKlp2 localized to oocyte microtubules. Time-lapse microscopy showed that disrupting MKlp2 expression with Paprotrain, a specific inhibitor of MKlp2, resulted in polar body extrusion failure. This could be rescued after rescuing oocytes from Paprotrain in fresh medium. Cell cycle analysis showed that most oocytes were arrested at metaphase I or telophase I. However, oocyte spindle structure and chromosome alignment were not disrupted after the inhibition of MKlp2 by Paprotrain. CONCLUSIONS: This study demonstrated that MKlp2 is crucial for oocyte maturation by regulating polar body extrusion.
Further investigation of Paprotrain: Towards the conception of selective and multi-targeted CNS kinase inhibitors.[Pubmed:27676471]
Eur J Med Chem. 2016 Nov 29;124:920-934.
Starting from a known compound, identified as the first inhibitor of the kinesin MKLP-2 and named Paprotrain, we have investigated its reactivity to produce through photochemistry a potent nanomolar inhibitor of the kinase DYRK1A. Using similar and different chemical pathways, we have designed several families of compounds that have been screened on a panel of five protein kinases: CK1delta/epsilon, CDK5/p25, GSK3alpha/beta, DYRK1A and CLK1, all involved in neurodegenerative disorders such as Alzheimer's disease. We have identified a first group of multi-targeted compounds, a second group of dual inhibitors of DYRK1A & CLK1 and a last group of selective inhibitors of CLK1. Then, our best submicromolar to nanomolar inhibitors were evaluated towards the closest members of the aforementioned kinases: DYRK1B and CLK4, as well as the subfamily CLK2-3. Several compounds appear to be particularly promising for the development of tools in the battle against Alzheimer's disease.
New MKLP-2 inhibitors in the paprotrain series: Design, synthesis and biological evaluations.[Pubmed:26778612]
Bioorg Med Chem. 2016 Feb 15;24(4):721-34.
Members of the kinesin superfamily are involved in key functions during intracellular transport and cell division. Their involvement in cell division makes certain kinesins potential targets for drug development in cancer chemotherapy. The two most advanced kinesin targets are Eg5 and CENP-E with inhibitors in clinical trials. Other mitotic kinesins are also being investigated for their potential as prospective drug targets. One recently identified novel potential cancer therapeutic target is the Mitotic kinesin-like protein 2 (MKLP-2), a member of the kinesin-6 family, which plays an essential role during cytokinesis. Previous studies have shown that inhibition of MKLP-2 leads to binucleated cells due to failure of cytokinesis. We have previously identified compound 1 (Paprotrain) as the first selective inhibitor of MKLP-2. Herein we describe the synthesis and biological evaluation of new analogs of 1. Our structure-activity relationship (SAR) study reveals the key chemical elements in the Paprotrain family necessary for MKLP-2 inhibition. We have successfully identified one MKLP-2 inhibitor 9a that is more potent than Paprotrain. In addition, in vitro analysis of a panel of kinesins revealed that this compound is selective for MKLP-2 compared to other kinesins tested and also does not have an effect on microtubule dynamics. Upon testing in different cancer cell lines, we find that the more potent Paprotrain analog is also more active than Paprotrain in 10 different cancer cell lines. Increased selectivity and higher potency is therefore a step forward toward establishing MKLP-2 as a potential cancer drug target.


